Tauvid has been approved by the U.S. Food and Drug Administration (FDA) in 2020 for positron emission tomography (PET) imaging of adult patients with cognitive impairments undergoing evaluation for Alzheimer’s disease (AD) based on tau pathology.
- tauvid™
- [18F]flortaucipir
- alzheimer’s disease
- PET imaging
1. Introduction
On the 28 May 2020, the U.S. Food and Drug Administration (FDA) approved Tauvid—a radioactive tracer—for positron emission tomography (PET) imaging of tau pathology in Alzheimer’s disease (AD) [1]. AD is a neurodegenerative disorder and the leading cause of dementia. According to the Alzheimer’s Disease International, it is estimated that over 50 million people worldwide have dementia, which is set to increase to over 150 million by 2050 [2]. Akin to many other neurodegenerative diseases, AD pathology is closely related to the accumulation of one or more folded or misfolded proteins. Two of the main hallmarks of AD are amyloid-β (Aβ) and tau, which cause the spread of extracellular Aβ plaques and intracellular tau neurofibrillary tangles (NFTs), respectively. Due to their evident appearance in AD patients, these have been the main focus in AD research as drug targets, as well as diagnostic targets [3].
Aβ tracers have been thoroughly investigated and the related research has grown exponentially in recent years. Between 2012 and 2014, [18F]florbetapir (Amyvid™), [18F]flutemetamol (Vizamyl™), and [18F]florbetaben (Neuroceq™) were consecutively approved by the FDA and the European Medicines Agency for imaging Aβ plaques in AD patients [8]. These PET tracers have greatly impacted the diagnosis of AD patients in the clinic and can assist in evaluating patients with cognitive impairment and dementia. Whilst most AD patients are positive for Aβ as indicated by Aβ PET tracers, there is still an unmet clinical need for a reliable, sensitive, and noninvasive tool to monitor disease progression as Aβ plaque deposition and cognitive impairment are poorly correlated in AD [9]. To further improve diagnostics and monitoring of disease progression, research has shifted increasingly towards the other main pathology: tau tangles.
Tau is a neuronal microtubule-associated protein that promotes microtubule self-assembly by tubulin and modulates the stability of axonal microtubules. The brain of an adult human contains six main isoforms of tau, where they are generated by alternative splicing of exons 2, 3, and 10. These isoforms are further categorised by whether they have a three or four carboxy-terminal repeat domains—which are referred to as 3R or 4R tau isoforms, respectively (Figure 1) [10].
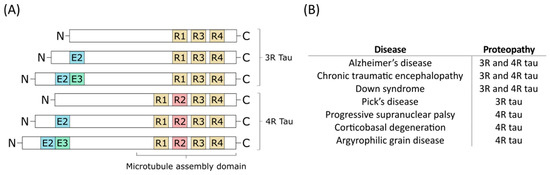
Figure 1. (A) The six different tau isoforms of an adult human. Exon 2 (E2), exon 3 (E3), and exon 10 (R2) are indicated in blue, green, and red, respectively. Alternative splicing creates the different isoforms which can be divided into 3-repeat (3R) tau and 4-repeat (4R) tau. (B) Different diseases can involve only 3R tau or 4R tau, or both of them as the main proteopathy [7].
2. Medicinal and Pharmaceutical Overview
2.1. Clinical Indication
The clinical indication of Tauvid is for estimating the density and distribution of NFTs in the brain of adult patients with cognitive impairments who are being evaluated for AD by PET [32].
Tauvid is explicitly not indicated in the labelling for the evaluation of patients for chronic traumatic encephalopathy (CTE). The differences in tau conformation and distribution may limit the binding of Tauvid and is therefore not indicated for CTE at the moment [32]—some further studies are discussed later.
2.2. Application
Tauvid might have a potential cardiotoxicity as it had an IC50 value of 0.610 μM in the in vitro hERG assay. Nevertheless, the safety margin is at least 42-fold when given a 20 µg dose [33], and therefore no cardiotoxicity is expected. Moreover, in vivo cardiovascular evaluation in dogs showed no evidence of QT prolongation. QT prolongation is a measure of delayed ventricular repolarisation, which means the heart muscle takes longer than normal to recharge between beats. Nonetheless, clinical trials exclude subjects with a history of risk factors for torsades de pointes and subjects taking drugs known to prolong the QT interval until more human cardiovascular safety data are available [33].
2.3. Pharmacology, Pharmacokinetics, and Pharmacodynamics
Tauvid is reported to have high affinity to immunopurified PHF-tau from postmortem human AD brain tissue with a KD value of 0.68 nM by homologous competition [33], and a KD value 0.57 nM with a Bmax of 309 pmol/mg protein as determined by a saturation binding experiment [32,33]. Using postmortem sections of the frontal lobe region of AD patients, a KD value of 15 nM was determined in an in vitro autoradiography-based saturation binding study [34]. Tauvid was reported to specifically bind to native tau aggregates in human brain sections, whereas in vitro autoradiography and immunostaining studies showed that binding correlates with tau but not with Aβ. Moreover, no KD could be determined for Aβ, supporting the selectivity of Tauvid for PHF-tau over Aβ [28]. Tauvid was assessed against a panel of CNS receptors, ion channels, transporters, enzymes, and human tissues by competitive binding and functional assays. For most of these targets, <50% inhibition of specific binding was achieved at concentration of 10 µM Tauvid, except for norepinephrine transporter, monoamine transporter (VMAT2), polyamine site on the glutamate receptor, µ-opiate receptor, and acetylcholinesterase. IC50 values were determined for the norepinephrine transporter, VMAT2, and polyamine site of the glutamate receptor: 2.2, 0.4, and 2.7 μM, respectively [28]. Despite that some inhibition is observed, these are far above the concentrations used for PET imaging.
Tauvid has a measured logP value of 1.67 and was found to efficiently cross the blood–brain barrier with a rapid brain penetration and fast washout [28]. After intravenous injection of Tauvid, it is distributed throughout the body with <10% of injected radioactivity present in the blood 5 min after administration, demonstrating a rapid blood clearance [32]. The clearance of Tauvid primarily occurs by hepatobiliary and renal excretion.
This entry is adapted from the peer-reviewed paper 10.3390/ph14020110
