Climate change affects the possibility of crop production and yield and disrupting the maintenance of crop biodiversity, including ornamentals. Warsaw is located in a temperate zone with mixed continental and oceanic climate influences. This research examines the response of once-blooming rambler roses to changing climate conditions in connection with their frost resistance and ornamental value. The 15 selected rambler rose cultivars were observed in the years 2000–2016 in the Polish Academy of Sciences Botanical Garden—Center for Biological Diversity Conservation in Powsin. Damage to shrubs caused by frost, the timing of bud break, leaf development, and initial, full, and final flowering were recorded. The changes in phenology and frost damage were the effect of weather conditions in the autumn–winter–spring period. Frost damage influenced the flowering and growth of plants in different ways, depending on the extent of required pruning. Their reintroduction helped to maintain biodiversity of old cultivars, which makes these roses a proposal for the lowlands of Central Europe.
1. Introduction
Knowledge of the phenology of wild and cultivated plants is important for horticultural crop production, meteorological sciences, and botany [
1,
2]. Plant phenology is a source of knowledge of periodic biological events affected by the environment and also the most reliable bioindicator [
3,
4]. It reflects biological and physical systems independently [
5]. Changes in springtime phenological events of perennial [
6,
7,
8] and woody plants have often been documented [
3,
5,
7,
9] and are more consistent in direction and magnitude than changes in summer and autumn phenophases [
4,
5,
7,
9,
10]. Zheng et al. [
5] selected 11 phenophases in nine woody species, namely, bud expansion, bud burst, first flowering, 50% of full flowering, end of flowering, first leaf, 50% of full leaf expansion, beginning of leaf coloring, end of leaf coloring, beginning of leaf fall, and end of leaf fall [
5]. The beginning dates of spring and summer phenophases advanced with time, while the start of autumn and winter phenophases became delayed. These changes were significantly correlated with temperature [
5]. Changes in climate can affect bud dormancy and cold hardiness, which are critical adaptations for the survival of winter cold stress by perennial plants of the temperate zone,
Vitis species among them [
11]. Bud dormancy allows perennial and woody plants to survive the winter in temperate climates [
12].
To examine the effects of climate change, botanical gardens make standard phenological observations of many plant taxa growing on their area limiting the number of factors that might alter long term changes [
13]. Their behavior can provide insight into how species will respond in the wild in terms of, e.g., changes in flowering, leaf-out times, and fruiting [
13]. The same principles can be applied to crops and ornamental plants. Botanical gardens located in large urban areas have tended to warm more rapidly than surrounding areas because of the urban heat island effect [
13]. Moreover, the effects of gas pollution on many plant traits, such as their phenology, seem minor in relation to the effects of temperature, light, and precipitation [
14]. Botanical gardens have a unique set of resources, including controlled growing conditions, which allows them to host important climate change research that cannot be easily undertaken elsewhere. Due to hosting large collections of plants from a wide range of areas, they are in the position to address many questions related to climate change that are often too difficult to examine at any other location [
15]. Observations in botanical gardens are recorded annually or periodically by experienced staff, and these records can also be used to track changes in anatomy or physiology [
13].
The rapid climatic changes observed over several dozens of years indicate that the average temperature has risen and the vegetation season has been slightly prolonged in the European area [
16]. These changes influence the diversity and distribution of species, and consequently ecosystems and biodiversity. It is estimated that ca. 32% of European plant species present in 1990 will disappear by 2050. These species take up 44% of the modeled European area. The distribution of natural species is projected to shift towards the north-east [
16]. Due to climatic change, some species will no longer be able to grow at their present locations due to lack of temperature tolerance, water stress, competition with other plant species, or changes in patterns of herbivory [
16,
17,
18].
The import of new species to nurseries and, in consequence, to the market poses a risk of their emergence as invasive species in the local environment and landscape, particularly in rural areas [
19]. The most decisive climate factors limiting the number of non-native plant species able to grow are not only frost resistance and autumn–winter–spring conditions but also the ability to grow in a changing climate [
19,
20]. During the years 2002–2016 1781 new species and cultivars were introduced in Polish nurseries associated with the Polish Nurserymen Association [
21]. Most of them come from a higher zone—6B in the USDA codification [
22]—and originate from, e.g., Western Europe or Asia [
19]. However, agriculture, horticulture, and forestry are the main sources of alien plant expansion. Such species can become invasive and lead to the extinction and reduction of native species [
23].
The use of cultivars in gardens limited the introduction of new potentially invasive species to the market by what is safer for maintaining the biodiversity of the environment; however, some are expansive, and their seed production should be evaluated in this regard. Their invasiveness needs to be considered on the basis of the evaluation of the entire life cycle of the cultivar and its offspring [
24].
Roses are one of the oldest cultivated crops [
25] and most important ornamental plants [
26,
27]; moreover, they have been significant in many fields of human life, both in Asia and in Europe, for thousands of years. The cultivated species, varieties, and cultivars of roses are arranged in several dozen groups in terms of origin and type of growth [
25]. Roses can be ordered in terms of type of growth into the following groups: Hybrid Teas, Floribundas, Polyanthas, Miniatures, Rambler and Climbers, Shrubs, and Ground Cover [
25,
28]. The classification of basic groups of historical rambler roses in terms of their origin includes, e.g., Hybrid Wichurana, Hybrid Setigera, and Hybrid Multiflora [
25,
28]. The healing properties of roses have been appreciated and documented for centuries. The production of petal oil, the most important rose ingredient used in perfumery and the cosmetic industry, is estimated to continue expansion [
3]. Rose oil also has applications in pharmacology, in which it is used for its anti-HIV, antibacterial, antioxidant, hypnotic, antidiabetic, and relaxant effects [
27]. Climbing roses are a very diverse group of varied origin and different decorative values [
21,
25,
28]. They are traditionally used in parks, courtyards, squares, estate greenery, and gardens. They do not require much space and are able to vine up walls in very narrow streets, right next to buildings and small courtyards [
21,
28] like other climbing plants [
29]. Roses planted in restaurant and café gardens make sitting at nearby tables more pleasant. The importance of roses as part of the greenery in the city is not to be questioned [
21,
28]. Moreover, most rose cultivars produce few or no seeds and are not expansive [
21,
25,
28].
2. Flowering
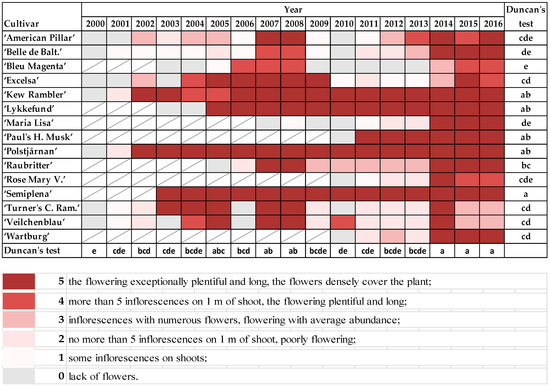
The rambler roses started flowering in the first days of June. “Maria Lisa”, “Paul’s Himalayan Musk”, and “Polstjårnan” were the first to start blooming; “Excelsa” and “Rose Mary Viaud” were the last (
Figure S4). If the shoots were damaged to the ground (points 6 and 7 on the scale), the low pruned shrubs did not flower, or only a few flowers appeared on old parts of shoots. Exceptionally plentiful and long flowering was observed in “Semiplena” and “Kew Rambler”, “Lykkefund”, “Paul’s Himalayan Musk”, and “Polstjårnan”. Low quality of flowering, especially after frosty winters, were noticed in “Bleu Magenta”, “American Pillar”, “Belle de Baltimore”, “Maria Lisa”, and “Rose Mary Viaud”. Moreover, the flowering was exceptionally plentiful after mild winters (2007, 2008, and 2014−2016) in all plants that were already a few years old ().
Figure 8. The abundance of flowering in rambler roses on the following scale: 0—lack of flowers; 1—some inflorescences on shoots; 2—no more than 5 inflorescences on 1 m of shoot, but flowering poorly; 3—inflorescences with numerous flowers, but flowering with average abundance; 4—more than 5 inflorescences on 1 m of shoot, plentiful and long flowering; 5—exceptionally plentiful and long flowering, the flowers densely cover the plant. Different letters indicate significant differences in the cultivars and the years. The Duncan’s test (α = 0.05) was used.
Correlation analysis of the ramblers for the timing of the start of flowering showed a strict relationship between the average temperature in winter and spring months for all cultivars. A decrease in the average temperature in March correlated with a later start to flowering in “American Pillar”, “Belle de Baltimore”, “Excelsa”, “Polstjårnan”, and “Raubritter”, while a decrease in the average temperature in April was connected with a later start to the flowering of “Kew Rambler” and “Veilchenblau” ().
Table 6. The part of matrices of effect correlations between average monthly air temperature (2006, 2010, and 2016) and the start of the flowering period (BBCH 60 601) in rambler roses.
| Cultivar |
SD |
Month |
| October |
November |
December |
January |
February |
March |
April |
| “American Pillar” |
9.87 |
0.971 *** |
−0.745 ** |
0.974 *** |
0.574 * |
0.414 |
−0.988 *** |
0.868 *** |
| “Belle de Baltimore” |
7.64 |
0.779 ** |
−0.957 *** |
0.978 *** |
0.176 |
−0.008 |
−0.960 *** |
0.996 *** |
| “Bleu Magenta” |
9.81 |
0.763 ** |
0.104 |
0.388 |
0.998 *** |
0.981 *** |
−0.455 |
0.106 |
| “Excelsa” |
11.55 |
0.179 |
−0.914 *** |
0.604 * |
−0.512 * |
−0.661 * |
−0.543 * |
0.808 ** |
| “Kew Rambler” |
2.52 |
−0.555 * |
0.998 *** |
−0.871 *** |
0.128 |
0.308 |
0.832 ** |
−0.976 *** |
| “Lykkefund” |
5.51 |
−0.701 ** |
−0.194 |
−0.303 |
−0.997 *** |
−0.995 *** |
0.373 |
−0.015 |
| “Maria Lisa” |
0.58 |
0.763 ** |
0.104 |
0.388 |
0.998 *** |
0.981 *** |
−0.455 |
0.106 |
| “Paul’s Him. Musk” |
4.04 |
0.763 ** |
0.104 |
0.388 |
0.998 *** |
0.981 *** |
−0.455 |
0.106 |
| “Polstjårnan” |
7.55 |
0.555 * |
−0.998 *** |
0.871 ** |
−0.128 |
−0.308 |
−0.832 ** |
0.976 *** |
| “Raubritter” |
6.51 |
0.612 * |
−0.998 *** |
0.903 *** |
−0.058 |
−0.240 |
−0.869 ** |
0.989 *** |
| “Rose Mary Viaud” |
8.66 |
0.763 ** |
0.104 |
0.388 |
0.998 *** |
0.981 *** |
−0.455 |
0.106 |
| “Semiplena” |
0.58 |
−0.763 ** |
−0.104 |
−0.388 |
−0.998 *** |
−0.981 *** |
0.455 |
−0.106 |
| “Turner’s Crim. Ram.” |
5.77 |
0.763 ** |
0.104 |
0.388 |
0.998 *** |
0.981 *** |
−0.455 |
0.106 |
| “Veilchenblau” |
2.52 |
−0.730 ** |
0.976 *** |
−0.960 *** |
−0.101 |
0.083 |
0.937 *** |
−0.998 *** |
| “Wartburg” |
0.58 |
0.763 ** |
0.104 |
0.388 |
0.998 *** |
0.981 *** |
−0.455 |
0.106 |
This entry is adapted from the peer-reviewed paper 10.3390/plants10030457