γ-Hydroxybutyric acid (GHB) is an endogenous short chain fatty acid that acts as a neurotransmitter and neuromodulator in the mammalian brain. It has often been illegally abused or misused due to its strong anesthetic effect, particularly in drug-facilitated crimes worldwide. However, proving its ingestion is not straightforward because of the difficulty in distinguishing between endogenous and exogenous GHB, as well as its rapid metabolism. Metabolomics and metabolism studies have recently been used to identify potential biomarkers of GHB exposure.
- γ-hydroxybutyrate
- drugs of abuse
- drug-facilitated crimes
- metabolomics
1. Introduction
Metabolomics is a field of omics science that investigates changes in metabolites with molecular weights of 1500 Da or less in biological samples and aims to understand metabolic pathways related to abnormal conditions or diseases. It is used to discover biomarkers that can serve as indicators of normal and pathological processes or reactions upon exposure to drugs, toxicants, or any intervention [1][2]. Analytical methods, including gas chromatography-mass spectrometry (GC-MS), liquid chromatography-mass spectrometry (LC-MS), nuclear magnetic resonance (NMR), and capillary electrophoresis-mass spectrometry (CE-MS), are used to investigate alterations in the concentrations of metabolites in a variety of biological samples such as blood, urine, and hair. In general, MS offers the advantage of displaying high sensitivity and a wide detection range, while NMR allows for non-destructive and minimal sample preparation [2][3]. Plasma and serum samples provide much information on physiological and pathological conditions in a particular biological system, over a short period of time [4]. Since many biogenic products eventually find their way to the urine, urinary metabolites are very beneficial for understanding the condition of diseases. Owing to the simple and noninvasive sampling process, the presence of metabolites in large quantities, and the extended detection window, urine is considered an ideal sample compared to plasma and serum for biomarker monitoring in clinical analyses [5][6]. Among biological samples, hair is more recently being used to monitor chronic drug use or chronic diseases, because it provides long detection windows, and segmental analysis of hair reflects the toxicological or pathological history [7]. Analytical approaches for metabolomics include targeted and untargeted analyses. Targeted analysis investigates metabolic changes based on physicochemically similar metabolomes (e.g., carbohydrates, amino acids (AAs), organic acids (OAs), nucleosides) or biochemical (e.g., glycolysis, gluconeogenesis, β-oxidation, or citric acid cycle) and metabolic (e.g., phase-I and -II metabolism) pathways. Therefore, selective and sensitive sample preparation methods and optimized analytical techniques are necessary for evaluating selected metabolites. Untargeted analysis is a method for analyzing the overall metabolic change in a selected biological sample, based on extensive information on unknown features, followed by the assignment of significantly altered features to specific metabolites. To investigate the maximum number of metabolites possible, non-selective approaches have been adopted for sample preparation and instrumental analysis [2][4]. In particular, metabolomics is currently being used to identify endogenous metabolites generated after exposure to addictive drugs [8][9][10][11]. This metabolomics approach can provide a new foundation for identifying effective diagnostic markers or therapeutic targets, if the knowledge of the mechanisms of a drug’s pharmacodynamic or pharmacokinetic properties is limited [12].
γ-Hydroxybutyric acid (4-hydroxybutyric acid, GHB, m.w. 104.1 g/mol) is a naturally occurring short-chain fatty acid in the human brain that acts as a neurotransmitter and neuromodulator [13]. GHB was developed as an anesthetic in the 1960s; however, its use was limited thereafter due to the occurrence of pain and delirium [14]. It is currently being used to treat Excessive Daytime Sleepiness (EDS) and cataplexy (Xyrem®; sodium oxybate) in patients with narcolepsy—with doses ranging from 3.0 to 9.0 g and treatment lasting from 4 to 8 weeks [15]—and for alcohol dependence and withdrawal (Alcover®) in Austria and Italy. However, as with other psychotropic drugs, there is a high risk of side effects upon GHB abuse, so its use other than for treatment of disease is strictly prohibited. Ingestion of GHB in recreational doses (>10 mg/kg) relieves tension, induces euphoria, and increases sexual pleasure. However, ingestion of 20–30 mg/kg of GHB can lead to dizziness, drowsiness, nausea, and vomiting, while intake of GHB over 50 mg/kg can lead to coma and death [16][17]. Moreover, people intoxicated with GHB may suffer from anterograde amnesia, which is one of the reasons criminals use this drug in sexual assault cases [18]. Possible withdrawal symptoms post GHB use include mild tremor, tachycardia, high blood pressure, anxiety, agitation, seizures, insomnia, severe disorientation, hallucinations, delirium, and rhabdomyolysis [19]. The average self-administration dose reported in dependent patients ranged from 32 to 67.2 g/day [20] to a maximum of 144 g/day [21] at 45-min to 2.5-h intervals. The severity of physical dependence on GHB is affected by the dose and duration of abuse [22], and high doses and/or long-term administration may be important factors influencing the physical dependence on GHB [23]. To date, a number of studies have been conducted to develop a therapeutic agent to reverse GHB-induced intoxication and sedative effects. Representatively, physostigmine has shown the possibility of recovering the GHB-induced change in consciousness state [24][25]. However, physostigmine increases cardiovascular-related side effects [26], while naloxone [27], an opiate antagonist, and flumazenil [28], a selective benzodiazepine receptor antagonist, have not been found to be effective in reversing the sedative effects of GHB. Upon studying whether the γ-aminobutyric acid (GABA)-B receptor antagonist has an effect on reducing mortality due to excessive GHB intake in mice, no significant effect was found [29]. Taken together, there is no antidote for GHB intoxication; in addition, the gap between the recreational and lethal doses of GHB is narrow, resulting in frequent accidental overdose deaths [30][31]. Therefore, various studies need to be carried out in clinical and forensic areas to diagnose and treat GHB poisoning and addiction. This review summarizes the methods and results of such studies, while focusing on studies of exogenous GHB (ExGHB) exploration using metabolomics or metabolite analysis, in addition to providing the latest information on the biomarkers of ExGHB. With specific focus on research publications from January 2010 till September 2020, a search was conducted in PubMed using the following keywords: [“γ-hydroxybutyric acid,” “γ-hydroxybutyrate,” or “GHB”] and [“metabolomics” or “metabolites”]. A total of 222 papers were searched with the keywords, and 25 papers were selected, excluding those with duplicate and low accuracy. Among them, 10 articles [32][33][34][35][36][37][38][39][40][41] were selected and analyzed after the exclusion of 14 papers. The exclusion criteria were as follows: reviews (n = 2) and purpose different from that of the current study (e.g., plant, succinic semialdehyde dehydrogenase deficiency, among others; n = 12). Additionally, one article [42] found in the reference list in one [34] of the searched articles was included since it is a metabolomics study of GHB.
2. Biosynthesis and Metabolism of GHB
Figure 1 illustrates the biosynthesis and metabolism of GHB and the potential bi-omarkers for GHB exposure. In the human brain, GHB (endogenous GHB; EnGHB) is produced from GABA by the action of GABA aminotransferase and succinic semialdehyde reductase. EnGHB concentration is the highest in the striatum, about 11–25 μM [43], and the lowest in certain areas of the cerebellum and cerebral cortex [44]. It is also found in the heart, liver, kidney, muscle, and brown fat, but its function in these organs is still unclear [45]. The generated GHB is converted to succinic semialdehyde (SSA) and converted back to GABA [46], or is metabolized to succinic acid and removed for use in energy metabolism through the Krebs cycle [47]. In addition, a small amount of GHB that is not metabolized is excreted in the urine [17]. Since GHB exists as a physiological compound in the human body, and ExGHB exhibits the same physiological effects using almost the same neurobiological pathway as EnGHB, it is difficult to distinguish between EnGHB and ExGHB [48][49].
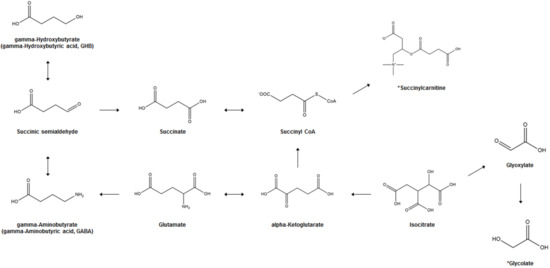
Figure 1. GHB biosynthesis and metabolism and potential biomarkers of GHB exposure. *; Proposed potential biomarker.
Another characteristic of GHB is that its metabolism occurs very quickly. A single oral intake (25 mg/kg body weight) of GHB in healthy adults results in the maximum plasma concentration within 30–90 min, with an average half-life of 40–60 min in the plasma [50]. GHB can be detected only within 6 h in the blood [51] and 12 h in the urine [52]. Because of these metabolic properties of GHB, it is very difficult to detect GHB in human biological samples to prove abuse or drug-related crime [53]. Until now, a number of studies have suggested cut-offs for the distinction of EnGHB and ExGHB concentrations in the urine or serum/plasma, but no consensus has been reached. Currently, 6–10 mg/L in urine and at least 4 mg/L in blood are accepted as typical human ExGHB cut-off concentrations [54][55][56]. However, some studies suggest a cut-off concentration of 1 mg/L in blood [57] and 5 mg/L [58][59] or 2 mg/L [60] in urine. Andresen et al. [61] collected blood and urine samples from 50 subjects who had never ingested GHB and analyzed them using GC-MS. The concentration of GHB ranged from 0.62–3.24 mg/L (mean = 1.14 mg/L and median = 0.97 mg/L) in serum and 0.64–4.20 mg/L (mean = 1.21 mg/L, median = 0.96 mg/L) in urine. Based on these results, it was suggested that the use of 6 mg/L for the urine GHB cut-off value instead of 10 mg/L is appropriate to avoid false negative interpretation. In addition, Kang et al. [62] reported that 0.09–1.8 g/mL of GHB was detected in the urine of 79 healthy volunteers, and when this concentration was adjusted to the creatine concentration, 4.5–530 g/mmol of GHB was present. Sex and age, but not smoking, alcohol, or caffeine intake, have been reported to have an effect on the concentration of EnGHB in urine. Despite the fact that multiple studies and discussions have been published on the cut-off level for GHB detection in biological specimens, the application of these methods to forensic cases is not simple. There are numerous factors to be considered, such as the in vivo and in vitro production of GHB in human biological specimens depending on the post-mortem interval, the time between sampling and analysis, the sample storage conditions (e.g., storage temperature, storage period, and addition of preservatives, among others), and the concomitant use of GHB with other drugs, including ethanol [63][64]. Although unusual, the conditions of GHBuria (γ-Hydroxybutyric aciduria) and succinic semialdehyde dehydrogenase deficiency (SSADH-D) should also be considered during diagnosis. SSADH-D is a disease caused by a mitochondrial enzyme that results in abnormal metabolism of the neurotransmitter GABA, leading to accumulation of GABA and GHB in the body, while GHBuria is a rare genetic disease characterized by the excretion of accumulated excess GHB in the urine [65]. Increased endogenous GABA and GHB levels through SSADH-D contribute to neurological disorders such as cognitive deficit and speech impairment, ataxia, hypotension, decreased reflexes, behavioral dysregulation, and compulsion [66].
3. GHB-Associated Metabolic Changes in AA, OA, and Polyamine (PA)
Table 1 summarizes metabolomics studies performed to investigate metabolic changes following GHB exposure in the urine of rats or humans [32][33][34][42]. GHB-associated metabolic changes in animal studies [32][34][42] were further confirmed in a clinical setting [33]. To perform the targeted metabolic profiling of AA, OA, and PA in rat urine, chemical derivatization was conducted for specific functional groups followed by GC-MS analysis. For untargeted metabolomics using NMR, human urine was lyophilized and reconstituted in D2O.
Table 1. Summary of metabolic changes in organic acids, amino acids, and polyamines following GHB exposure.
| Reference No. | Sample | Sample Preparation | Analytical Platform (Untargeted or Targeted) |
Treatment (Administration Dose, Route, and No. of Doses) |
Sampling Time | Metabolic Changes | Summary |
|---|---|---|---|---|---|---|---|
| [42] | Male SD rat (n = 18), urine | Methoxime/tert-butyldimethylsilyl derivatization | GC-SIM-MS(targeted) | 600 mg/kg GHB, i.p. once per day for 10 days | For 12 h following single administration | Pyruvic acid (↑), acetoacetic acid (↓), lactic acid (↑), glycolic acid (↑), 2-hydroxybutyric acid (↓), malonic acid (↓), succinic acid (↑), fumaric acid (↑), oxaloacetic acid (↑), malic acid (↑), α-ketoglutaric acid (↑), 2-hydroxyglutaric acid (↑), cis-aconitic acid (↑), citric acid (↑), isocitric acid (↑), γ-hydroxybutyric acid (↑) | Alteration of organic acid metabolism related with tricarboxylic acid cycle Key metabolite: 2-hydroxyglutaric acid |
| For 12 h following multiple administration (10 times) | Pyruvic acid (↑), acetoacetic acid (↑), lactic acid (↑), glycolic acid (↑), 2-hydroxybutyric acid (↑), malonic acid (↑), succinic acid (↑), fumaric acid (↑), oxaloacetic acid (↑), malic acid (↑), α-ketoglutaric acid (↑), 2-hydroxyglutaric acid (↑), cis-aconitic acid (↑), citric acid (↑), isocitric acid (↑), γ-hydroxybutyric acid (↑) | ||||||
| [34] | Male SD rat (n = 25), urine | Ethoxycarbonyl/tert-butyldimethylsilyl derivatization | GC-SIM-MS(targeted) | 600 mg/kg GHB, i.p. once per day for 10 days | For 12 h following single administration | Alanine (↑), glycine (↑), α-aminobutyric acid (↑), valine (↑), β-aminoisobutyric acid (↑), leucine, isoleucine (↑), serine (↑), proline (↑), γ-aminobutyric acid (↑), pipecolic acid (↑), 4-hydroxyproline (↑), methionine (↑), phenylalanine (↑), aspartic acid (↑), glutamic acid (↑), asparagine (↑), ornithine (↑), lysine (↑), histidine (↑), tyrosine (↑), tryptophan (↑), glutamine (↓) | Alteration of amino acid metabolism Key metabolite(s): phenylalanine, glutamic acid, aspartic acid, asparagine, and methionine |
| For 12 h following multiple administration (10 times) | Leucine (↑), isoleucine (↑), serine (↑), proline (↑), histidine (↓), phenylalanine (↓), γ-aminobutyric acid (↑), pyroglutamic acid (↑), α-aminoadipic acid (↑), glycine (↓), methionine (↓), tyrosine (↓) 4-hydroxyproline (↓), aspartic acid (↓), glutamic acid (↓), asparagine (↓), ornithine (↓), glutamine (↓), lysine (↓) | ||||||
| [32] | Male SD rat (n = 18), urine | N-ethoxylcarbonyl-N-pentafluoropropionyl derivatization | GC-SIM-MS(targeted) | 600 mg/kg GHB, i.p. once per day for 10 days | For 12 h following single administration | Putrescine (↑), N1-acetylspermidine (↑), spermine (↑), N1-acetylspermine (↑) |
Alteration of polyamine metabolism Key metabolite(s): N1-acetylspermine and spermine |
| For 12 h following multiple administration (10 times) | Putrescine (↓), N1-acetylspermidine (↓), spermine (↑), N1-acetylspermine (↑) |
||||||
| [33] | Healthy men & women (n = 12, each), urine | Lyophilization and reconstitution in D2O | NMR(untargeted) | 25 mg/kg GHB (Xyrem®) | Urine: 10 min, 1, 2, 4, 6, 14, 20, 24, and 30 h post dose | Glycolate (↑), succinate (↑) |
Confirmation of glycolate and succinate as potent markers for GHB, Slower elimination of glycolate (even after 24 h) than succinate (at time point of 6 h) |
GC, gas chromatography; SIM, selected ion monitoring; MS, mass spectrometry; i.p., intraperitoneal injection; ↑, significantly increased vs. vehicle group; ↓, significantly decreased vs. vehicle group.
A previous study demonstrated the accumulation of D-2-hydroxyglutaric acid (D-2-HG) in blood and urine from baboons following GHB exposure, based on the hypothesis of conversion of GHB to D-2-HG, a reaction catalyzed by d-2-hydroxyglutarate transhydrogenase [67]. In the intermediate metabolic pathway, various OAs such as mono-, di-, and tri-carboxylic acids with hydroxyl, aromatic rings, and carbonyl groups as metabolites are involved in the metabolism of AAs as well as the tricarboxylic acid (TCA) cycle and β-oxidation of fatty acids. According to previous studies, changes in OA levels are reported in the conditions of diabetes [68], cancer [69] and genetic metabolic disorders [70][71]. An animal study was performed to study the effect of GHB administration for 1 or 10 days (600 mg/kg, i.p., once/day) on TCA cycle-related OA and D-2-HG levels in rats using GC-MS analysis. The results showed that single or multiple doses of GHB increased most of the OA, including GHB and D-2-HG, in the urine of rats (p < 0.05); in addition, the single dose group showed a greater increase in these factors than the multiple dose group. From these results, it was concluded that citrate, isocitrate, and cis-aconitate profiling are useful biomarkers for discriminating GHB intoxication [42]. AAs are precursors and products of OAs that are closely related to GHB metabolism and the TCA cycle; AA in the blood gets excreted into urine to maintain homeostasis. Therefore, profiling of AAs using urine samples is very useful for monitoring altered metabolism and understanding biochemical changes [71][72]. Seo et al. [34] profiled AAs, including GABA and glutamic acid, using GC-MS after GHB administration (600 mg/kg, i.p., once/day) to rats for 1 or 10 days. They found that 28 AAs were detected in urine in the control and GHB-intake groups at levels exceeding the limit of quantification of the AA profiling method. In addition, levels of GABA and glutamic acid, which are GHB metabolites, were significantly higher in the urine of the single treatment group. In addition, it has been reported that phenylalanine, glutamic acid, aspartic acid, asparagine, and methionine are metabolites that can help in distinguishing whether or not GHB has been taken and the number of doses. These results suggest that changes in AA metabolism could be used as a useful biomarker for discriminating GHB administration. PA is being studied with a focus on cell growth, cell proliferation, protein, and nucleic acid synthesis by controlling its acetylation-deacetylation, according to changes in biochemical conditions [73][74][75]. Recently, studies on PA have focused on its role in a PA-mediated stress response signaling system and as a marker for monitoring and diagnosing various disease states [76][77][78]. Therefore, endogenous PA profile analysis is important for understanding biochemical changes post GHB exposure. Accordingly, Lee et al. [32] administered GHB (600 mg/kg, i.p., once/day) to rats for 1 or 10 days and then profiled PA in the urine using GC-MS. The levels of N1-acetylspermine, putrescine, N1-acetylspermidine, and spermine were found to be significantly higher in the single administration group, as compared to the control group, but the levels of putrescine and N1-acetylspermidine were found to be significantly lower in the multiple administration group. In summary, there were significant differences in the levels of N1-acetylspermine between the three groups, while the levels of spermine differed significantly between the administered and non-administered groups. Therefore, N1-acetylspermine and spermine have been suggested as potential biomarkers of GHB exposure and poisoning.
Although little metabolomics studies have been conducted on clinical settings, the elevated urinary levels of OAs, such as succinate, following GHB exposure, were observed in a previous human study [33]. Palomino-Schätzlein et al. [33] collected urine samples for 30 h after one dose of GHB (Xyrem®, sodium oxybate, 500 mg/L) in healthy adult men and women and analyzed them using NMR spectroscopy. There was a significant increase in the concentration of succinate and glycolate in urine post GHB administration. However, while the levels of succinate decreased rapidly, glycolate was identified as a biomarker capable of discriminating ExGHB, as it maintained a high level for more than 20 h, as compared to the levels before GHB intake.
This entry is adapted from the peer-reviewed paper 10.3390/metabo11020101
References
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221.
- Cambiaghi, A.; Ferrario, M.; Masseroli, M. Analysis of metabolomic data: Tools, current strategies and future challenges for omics data integration. Brief. Bioinform. 2017, 18, 498–510.
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459.
- Bujak, R.; Struck-Lewicka, W.; Markuszewski, M.J.; Kaliszan, R. Metabolomics for laboratory diagnostics. J. Pharm. BioMed. Anal. 2015, 113, 108–120.
- Kim, M.; Jang, W.J.; Shakya, R.; Choi, B.; Jeong, C.H.; Lee, S. Current Understanding of Methamphetamine-associated metabolic changes revealed by the metabolomics approach. Metabolites 2019, 9, 195.
- Choi, B.; Kim, S.P.; Hwang, S.; Hwang, J.; Yang, C.H.; Lee, S. Metabolic characterization in urine and hair from a rat model of methamphetamine self- administration using LC-QTOF-MS-based metabolomics. Metabolomics 2017, 13, 119.
- Jang, W.J.; Choi, J.Y.; Park, B.; Seo, J.H.; Seo, Y.H.; Lee, S.; Jeong, C.H. Hair Metabolomics in Animal Studies and Clinical Settings. Molecules 2019, 24, 2195.
- Kim, S.; Jang, W.J.; Yu, H.; Kim, J.; Lee, S.K.; Jeong, C.H.; Lee, S. Revealing Metabolic Perturbation Following Heavy Methamphetamine Abuse by Human Hair Metabolomics and Network Analysis. Int. J. Mol. Sci. 2020, 21, 6041.
- Kim, S.; Jang, W.J.; Yu, H.; Ryu, I.S.; Jeong, C.H.; Lee, S. Integrated Non-targeted and Targeted Metabolomics Uncovers Dynamic Metabolic Effects during Short-Term Abstinence in Methamphetamine Self-Administering Rats. J. Proteome Res. 2019, 18, 3913–3925.
- Nielsen, K.L.; Telving, R.; Andreasen, M.F.; Hasselstrøm, J.B.; Johannsen, M. A Metabolomics Study of Retrospective Forensic Data from Whole Blood Samples of Humans Exposed to 3,4-Methylenedioxymethamphetamine: A New Approach for Identifying Drug Metabolites and Changes in Metabolism Related to Drug Consumption. J. Proteome Res. 2016, 15, 619–627.
- Zaitsu, K.; Miyawaki, I.; Bando, K.; Horie, H.; Shima, N.; Katagi, M.; Tatsuno, M.; Bamba, T.; Sato, T.; Ishii, A.; et al. Metabolic profiling of urine and blood plasma in rat models of drug addiction on the basis of morphine, methamphetamine, and cocaine-induced conditioned place preference. Anal. BioAnal. Chem. 2014, 406, 1339–1354.
- Alvarez, J.A.; Chong, E.Y.; Walker, D.I.; Chandler, J.D.; Michalski, E.S.; Grossmann, R.E.; Uppal, K.; Li, S.; Frediani, J.K.; Tirouvanziam, R.; et al. Plasma metabolomics in adults with cystic fibrosis during a pulmonary exacerbation: A pilot randomized study of high-dose vitamin D(3) administration. Metabolism 2017, 70, 31–41.
- Kamal, R.M.; van Iwaarden, S.; Dijkstra, B.A.; de Jong, C.A. Decision rules for GHB (γ-hydroxybutyric acid) detoxification: A vignette study. Drug Alcohol Depend. 2014, 135, 146–151.
- Kam, P.C.; Yoong, F.F. Gamma-hydroxybutyric acid: An emerging recreational drug. Anaesthesia 1998, 53, 1195–1198.
- Boscolo-Berto, R.; Viel, G.; Montagnese, S.; Raduazzo, D.I.; Ferrara, S.D.; Dauvilliers, Y. Narcolepsy and effectiveness of gamma-hydroxybutyrate (GHB): A systematic review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 2012, 16, 431–443.
- Schep, L.J.; Knudsen, K.; Slaughter, R.J.; Vale, J.A.; Mégarbane, B. The clinical toxicology of γ-hydroxybutyrate, γ-butyrolactone and 1,4-butanediol. Clin. Toxicol. (Phila) 2012, 50, 458–470.
- Snead, O.C., 3rd; Gibson, K.M. Gamma-hydroxybutyric acid. N. Engl. J. Med. 2005, 352, 2721–2732.
- Kapitány-Fövény, M.; Zacher, G.; Posta, J.; Demetrovics, Z. GHB-involved crimes among intoxicated patients. Forensic Sci. Int. 2017, 275, 23–29.
- Zvosec, D.L.; Smith, S.W.; Porrata, T.; Strobl, A.Q.; Dyer, J.E. Case series of 226 γ-hydroxybutyrate-associated deaths: Lethal toxicity and trauma. Am. J. Emerg. Med. 2011, 29, 319–332.
- de Jong, C.A.; Kamal, R.; Dijkstra, B.A.; de Haan, H.A. Gamma-hydroxybutyrate detoxification by titration and tapering. Eur. Addict. Res. 2012, 18, 40–45.
- Dyer, J.E.; Roth, B.; Hyma, B.A. Gamma-hydroxybutyrate withdrawal syndrome. Ann. Emerg. Med. 2001, 37, 147–153.
- Craig, K.; Gomez, H.F.; McManus, J.L.; Bania, T.C. Severe gamma-hydroxybutyrate withdrawal: A case report and literature review. J. Emerg. Med. 2000, 18, 65–70.
- Weerts, E.M.; Goodwin, A.K.; Griffiths, R.R.; Brown, P.R.; Froestl, W.; Jakobs, C.; Gibson, K.M. Spontaneous and precipitated withdrawal after chronic intragastric administration of gamma-hydroxybutyrate (GHB) in baboons. Psychopharmacology 2005, 179, 678–687.
- Caldicott, D.G.; Kuhn, M. Gamma-hydroxybutyrate overdose and physostigmine: Teaching new tricks to an old drug? Ann. Emerg. Med. 2001, 37, 99–102.
- Yates, S.W.; Viera, A.J. Physostigmine in the treatment of gamma-hydroxybutyric acid overdose. Mayo Clin. Proc. 2000, 75, 401–402.
- Zvosec, D.L.; Smith, S.W.; Litonjua, R.; Westfal, R.E. Physostigmine for gamma-hydroxybutyrate coma: Inefficacy, adverse events, and review. Clin. Toxicol. 2007, 45, 261–265.
- Devoto, P.; Colombo, G.; Cappai, F.; Gessa, G.L. Naloxone antagonizes ethanol- but not gamma-hydroxybutyrate-induced sleep in mice. Eur. J. Pharm. 1994, 252, 321–324.
- Lee, D.C.; Satz, W.A.; Dougherty, T.; Greene, T. An investigation of flumazenil to antagonize gamma-hydroxybutyrate intoxication in a murine model. J. Med. Toxicol. 2006, 2, 68–70.
- Carai, M.A.; Colombo, G.; Gessa, G.L. Resuscitative effect of a gamma-aminobutyric acid B receptor antagonist on gamma-hydroxybutyric acid mortality in mice. Ann. Emerg. Med. 2005, 45, 614–619.
- Elliott, S. Nonfatal instances of intoxication with gamma-hydroxybutyrate in the United Kingdom. Drug Monit. 2004, 26, 432–440.
- Kugelberg, F.C.; Holmgren, A.; Eklund, A.; Jones, A.W. Forensic toxicology findings in deaths involving gamma-hydroxybutyrate. Int. J. Leg. Med. 2010, 124, 1–6.
- Lee, H.-S.; Seo, C.; Kim, Y.-A.; Park, M.; Choi, B.; Ji, M.; Lee, S.; Paik, M.-J. Metabolomic study of polyamines in rat urine following intraperitoneal injection of γ-hydroxybutyric acid. Metabolomics 2019, 15, 58.
- Palomino-Schätzlein, M.; Wang, Y.; Brailsford, A.D.; Parella, T.; Cowan, D.A.; Legido-Quigley, C.; Pérez-Trujillo, M. Direct Monitoring of Exogenous γ-Hydroxybutyric Acid in Body Fluids by NMR Spectroscopy. Anal. Chem. 2017, 89, 8343–8350.
- Seo, C.; Na, M.; Jang, J.; Park, M.; Choi, B.; Lee, S.; Paik, M.-J. Monitoring of altered amino acid metabolic pattern in rat urine following intraperitoneal injection with γ-hydroxybutyric acid. Metabolomics 2018, 14, 111.
- Hanisch, S.; Stachel, N.; Skopp, G. A potential new metabolite of gamma-hydroxybutyrate: Sulfonated gamma-hydroxybutyric acid. Int. J. Leg. Med. 2016, 130, 411–414.
- Petersen, I.N.; Tortzen, C.; Kristensen, J.L.; Pedersen, D.S.; Breindahl, T. Identification of a new metabolite of GHB: Gamma-hydroxybutyric acid glucuronide. J. Anal. Toxicol. 2013, 37, 291–297.
- Pascali, J.P.; Fais, P.; Vaiano, F.; Ciolini, A.; Bertol, E. Zwitterionic HILIC stationary phase as a valuable alternative in separative techniques: Application to the analysis of gamma-hydroxybutyric acid and its metabolite in hair. J. Chromatogr. B Anal. Technol. BioMed. Life Sci. 2019, 1134–1135, 121876.
- Dias, A.S.; Castro, A.L.; Melo, P.; Tarelho, S.; Domingues, P.; Franco, J.M. A fast method for GHB-GLUC quantitation in whole blood by GC-MS/MS (TQD) for forensic purposes. J. Pharm. BioMed. Anal. 2018, 150, 107–111.
- Busardò, F.P.; Gottardi, M.; Tini, A.; Mortali, C.; Giorgetti, R.; Pichini, S. Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry Assay for Determination of Endogenous GHB and GHB-Glucuronide in Nails. Molecules 2018, 23, 2686.
- Piper, T.; Mehling, L.M.; Spottke, A.; Heidbreder, A.; Young, P.; Madea, B.; Hess, C.; Schänzer, W.; Thevis, M. Potential of GHB phase-II-metabolites to complement current approaches in GHB post administration detection. Forensic Sci. Int. 2017, 279, 157–164.
- Steuer, A.E.; Raeber, J.; Steuer, C.; Boxler, M.I.; Dornbierer, D.A.; Bosch, O.G.; Quednow, B.B.; Seifritz, E.; Kraemer, T. Identification of new urinary gamma-hydroxybutyric acid markers applying untargeted metabolomics analysis following placebo-controlled administration to humans. Drug Test Anal. 2019, 11, 813–823.
- Seo, C.; Park, M.; Choi, B.; Lee, S.; Paik, M.-J. Metabolomic analysis of urinary organic acids following intraperitoneal injection with γ-hydroxybutyric acid in rats. Metabolomics 2016, 12, 190.
- Tunnicliff, G. Sites of action of gamma-hydroxybutyrate (GHB)--a neuroactive drug with abuse potential. J. Toxicol. Clin. Toxicol. 1997, 35, 581–590.
- Wong, C.G.; Chan, K.F.; Gibson, K.M.; Snead, O.C. Gamma-hydroxybutyric acid: Neurobiology and toxicology of a recreational drug. Toxicol. Rev. 2004, 23, 3–20.
- Mamelak, M. Gammahydroxybutyrate: An endogenous regulator of energy metabolism. NeuroSci. Biobehav. Rev. 1989, 13, 187–198.
- Maitre, M. The gamma-hydroxybutyrate signalling system in brain: Organization and functional implications. Prog. NeuroBiol. 1997, 51, 337–361.
- Hogema, B.M.; Gupta, M.; Senephansiri, H.; Burlingame, T.G.; Taylor, M.; Jakobs, C.; Schutgens, R.B.; Froestl, W.; Snead, O.C.; Diaz-Arrastia, R.; et al. Pharmacologic rescue of lethal seizures in mice deficient in succinate semialdehyde dehydrogenase. Nat. Genet. 2001, 29, 212–216.
- Andresen, H.; Aydin, B.E.; Mueller, A.; Iwersen-Bergmann, S. An overview of gamma-hydroxybutyric acid: Pharmacodynamics, pharmacokinetics, toxic effects, addiction, analytical methods, and interpretation of results. Drug Test Anal. 2011, 3, 560–568.
- LeBeau, M.A.; Miller, M.L.; Levine, B. Effect of storage temperature on endogenous GHB levels in urine. Forensic Sci. Int. 2001, 119, 161–167.
- Brenneisen, R.; Elsohly, M.A.; Murphy, T.P.; Passarelli, J.; Russmann, S.; Salamone, S.J.; Watson, D.E. Pharmacokinetics and excretion of gamma-hydroxybutyrate (GHB) in healthy subjects. J. Anal. Toxicol. 2004, 28, 625–630.
- Kintz, P.; Goullé, J.P.; Cirimele, V.; Ludes, B. Window of detection of gamma-hydroxybutyrate in blood and saliva. Clin. Chem. 2001, 47, 2033–2034.
- Haller, C.; Thai, D.; Jacob, P., 3rd; Dyer, J.E. GHB urine concentrations after single-dose administration in humans. J. Anal. Toxicol. 2006, 30, 360–364.
- Busardò, F.P.; Pichini, S.; Zaami, S.; Pacifici, R.; Kintz, P. Hair testing of GHB: An everlasting issue in forensic toxicology. Clin. Chem. Lab. Med. 2018, 56, 198–208.
- Bosman, I.J.; Lusthof, K.J. Forensic cases involving the use of GHB in The Netherlands. Forensic Sci. Int. 2003, 133, 17–21.
- Elian, A.A. Determination of endogenous gamma-hydroxybutyric acid (GHB) levels in antemortem urine and blood. Forensic Sci. Int. 2002, 128, 120–122.
- Yeatman, D.T.; Reid, K. A study of urinary endogenous gamma-hydroxybutyrate (GHB) levels. J. Anal. Toxicol. 2003, 27, 40–42.
- Steinecke, H. Beitrag zur Bewertung von Gamma-Hydroxybuttersa¨ure (GHB)-Konzentrationen im Blut lebender Personen sowie in postmortalen Blutproben. ToxiChem. Krimtech. 2007, 74, 150–154.
- Crookes, C.E.; Faulds, M.C.; Forrest, A.R.; Galloway, J.H. A reference range for endogenous gamma-hydroxybutyrate in urine by gas chromatography-mass spectrometry. J. Anal. Toxicol. 2004, 28, 644–649.
- McCusker, R.R.; Paget-Wilkes, H.; Chronister, C.W.; Goldberger, B.A. Analysis of gamma-hydroxybutyrate (GHB) in urine by gas chromatography-mass spectrometry. J. Anal. Toxicol. 1999, 23, 301–305.
- Kavanagh, P.V.; Kenny, P.; Feely, J. The urinary excretion of gamma-hydroxybutyric acid in man. J. Pharm. Pharm. 2001, 53, 399–402.
- Andresen, H.; Sprys, N.; Schmoldt, A.; Mueller, A.; Iwersen-Bergmann, S. Gamma-hydroxybutyrate in urine and serum: Additional data supporting current cut-off recommendations. Forensic Sci. Int. 2010, 200, 93–99.
- Kang, S.; Oh, S.M.; Chung, K.H.; Lee, S. A surrogate analyte-based LC-MS/MS method for the determination of γ-hydroxybutyrate (GHB) in human urine and variation of endogenous urinary concentrations of GHB. J. Pharm. BioMed. Anal. 2014, 98, 193–200.
- White, C.M. Pharmacologic, pharmacokinetic, and clinical assessment of illicitly used gamma-hydroxybutyrate. J. Clin. Pharm. 2017, 57, 33–39.
- Busardo, F.P.; Kyriakou, C. GHB in biological specimens: Which cut-off levels should be taken into consideration in forensic toxicological investigation? Recent Pat. Biotechnol. 2014, 8, 206–214.
- Vogel, K.R.; Ainslie, G.R.; Walters, D.C.; McConnell, A.; Dhamne, S.C.; Rotenberg, A.; Roullet, J.B.; Gibson, K.M. Succinic semialdehyde dehydrogenase deficiency, a disorder of GABA metabolism: An update on pharmacological and enzyme-replacement therapeutic strategies. J. Inherit. Metab. Dis. 2018, 41, 699–708.
- DiBacco, M.L.; Roullet, J.B.; Kapur, K.; Brown, M.N.; Walters, D.C.; Gibson, K.M.; Pearl, P.L. Age-related phenotype and biomarker changes in SSADH deficiency. Ann. Clin. Transl. Neurol. 2019, 6, 114–120.
- Struys, E.A.; Verhoeven, N.M.; Jansen, E.E.; Ten Brink, H.J.; Gupta, M.; Burlingame, T.G.; Quang, L.S.; Maher, T.; Rinaldo, P.; Snead, O.C.; et al. Metabolism of gamma-hydroxybutyrate to d-2-hydroxyglutarate in mammals: Further evidence for d-2-hydroxyglutarate transhydrogenase. Metabolism 2006, 55, 353–358.
- Liebich, H.M. Gas chromatographic profiling of ketone bodies and organic acids in diabetes. J. Chromatogr. 1986, 379, 347–366.
- Hur, H.; Paik, M.J.; Xuan, Y.; Nguyen, D.T.; Ham, I.H.; Yun, J.; Cho, Y.K.; Lee, G.; Han, S.U. Quantitative measurement of organic acids in tissues from gastric cancer patients indicates increased glucose metabolism in gastric cancer. PLoS ONE 2014, 9, e98581.
- Kuhara, T. Gas chromatographic-mass spectrometric urinary metabolome analysis to study mutations of inborn errors of metabolism. Mass Spectrom. Rev. 2005, 24, 814–827.
- Paik, M.J.; Lee, H.J.; Kim, K.R. Simultaneous retention index analysis of urinary amino acids and carboxylic acids for graphic recognition of abnormal state. J. Chromatogr. B Anal. Technol. BioMed. Life Sci. 2005, 821, 94–104.
- Kim, J.-W.; Lee, G.; Moon, S.-M.; Park, M.-J.; Hong, S.K.; Ahn, Y.-H.; Kim, K.-R.; Paik, M.-J. Metabolomic screening and star pattern recognition by urinary amino acid profile analysis from bladder cancer patients. Metabolomics 2010, 6, 202–206.
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406.
- Seiler, N. Catabolism of polyamines. Amino Acids 2004, 26, 217–233.
- Tabor, C.W.; Tabor, H. Polyamines. Annu. Rev. BioChem. 1984, 53, 749–790.
- Gross, J.A.; Turecki, G. Suicide and the polyamine system. CNS Neurol. Disord. Drug Targets 2013, 12, 980–988.
- Nowotarski, S.L.; Woster, P.M.; Casero, R.A., Jr. Polyamines and cancer: Implications for chemotherapy and chemoprevention. Expert Rev. Mol. Med. 2013, 15, e3.
- Paik, M.J.; Kim, H.S.; Lee, Y.S.; Choi, H.D.; Pack, J.K.; Kim, N.; Ahn, Y.H. Metabolomic study of urinary polyamines in rat exposed to 915 MHz radiofrequency identification signal. Amino Acids 2016, 48, 213–217.
