Exosomes are lipid membrane-enclosed vesicles released by all cell types that act at the paracrine or endocrine level to favor cell differentiation, tissue homeostasis, organ remodeling, and immune regulation. Their biosynthesis begins with a cell membrane invagination which generates an early endosome that matures to a late endosome. By inward budding of the late endosome membrane, a multivesicular body (MVB) with intraluminal vesicles (ILVs) is generated. The fusion of MVBs with the plasma membrane releases ILVs into the extracellular space as exosomes, ranging in size from 30 to 100 nm in diameter. The bilipid exosome membrane is rich in cholesterol, ceramides, and phosphatidylserine and can be loaded with DNA, RNA, microRNAs, proteins, and lipids. It has been demonstrated that exosome secretion is a common mechanism used by the tumor to generate an immunosuppressive microenvironment that favors cancer development and progression, allowing tumor escape from immune control.
- extracellular vesicles
- exosomes
- cancer
- immunology
1. Exosome Biogenesis
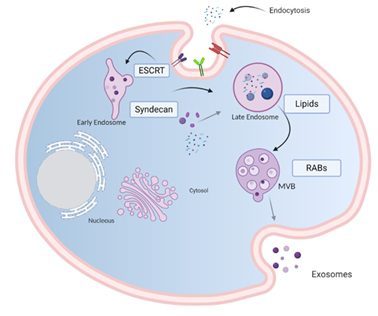
Exosomes are generated from late endosome membrane by an unconventional inward budding that results in the creation of large MVBs that act as a sorting platform for membrane proteins to develop ILVs loaded by the endosomal sorting complex required for transport (ESCRT) [9]. ILVs may follow one of three destinations: (i) if MVBs fuse with lysosomes, they are degraded; (ii) they could contribute to the development of specialized organelles such as melanosomes [10]; (iii) if the MVBs fuse with the plasma membrane, ILVs are secreted as exosomes into the extracellular milieu [11].
Several lipids and lipid metabolizing enzymes are involved in the formation and release of exosomes. Ceramide microdomains in areas with high sphingolipid concentrations can bind and generate large ceramide-rich domains to promote membrane budding [12,13]. Although the mechanism by which molecules are charged in exosomes is not clear, it is known that heparan sulfate proteoglycans, especially syndecans and their cytoskeleton coupling proteins, syntenins, appear to be involved in inducing intraluminal budding of the endosome membrane. The syntenin exosomes depend on the availability of heparan sulfate, syndecans, ALG2 interaction protein X (ALIX), and the endosomal classification complex required for transport (ESCRT) [14], which regulates the germination of the membrane on the cell surface and in the late endosome.
Loading molecules in the ILVs and exosome release from the endosomal membrane are mediated by two pathways: an endosomal sorting complex required for transport (ESCRT)-dependent pathway [15] and an ESCRT-independent pathway [16]. The ESCRT pathway comprises five different protein complexes (ESCRTs -0, -I, -II, and -III, and Vps4), capable of recognizing and classifying ubiquitinated load. ESCRT proteins are involved in the sequestration and classification of ubiquitous membrane proteins to deform the endosomal boundary membrane inward and generate the MVBs, while the Vps4 complex is needed to deliver the load to the vesicle (Figure 1) [17].
Figure 1. Model of biogenesis and release of exosomes. Cell-surface proteins and soluble molecules from the extracellular milieu enter cytoplasm by endocytosis or by plasma membrane invagination that results, at the luminal side of the cell, in the early-sorting endosome development which matures in a late-sorting endosome. Eventually, this late endosome generates MVBs by inward invagination of the endosomal membrane. This double invagination of the plasma membrane results in MVBs containing several ILVs. The MVBs can fuse with lysosomes where their cargo is degraded or can dock on the luminal side of the plasma membrane and merge with the plasma membrane to release the contained ILVs as exosomes. Rab GTPases, ESCRT, CD9, CD81, CD63, TSG101, ceramide, and Alix are involved in exosome biogenesis. Tetraspanins, integrins, sphingomyelinase, ceramides, and immunomodulatory proteins are exosome surface proteins. The molecular exosomes’ cargo is composed of cell surface proteins, intracellular proteins, RNA, DNA, amino acids, and metabolites. Image created with biorender.com.
2. General Composition of Exosomes
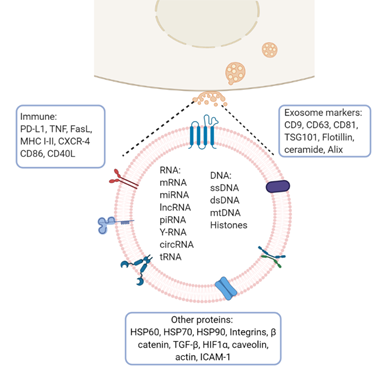
Exosomes from multiple organisms harbor more than 4500 proteins, 200 lipids, 1600 mRNAs, and 800 miRNAs [18]. They are present in bodily fluids and interact with adjacent cells inducing a variety of downstream effects which depend on the cell type from which they are derived and on their load-bearing molecules (membrane and cytosolic proteins, lipids, mRNA, and miRNA). The composition of exosomes varies depending on the originating cell type. In general, they are surrounded by a lipid bilayer enriched with sphingomyelin, phosphatidylserine, GM3 ganglioside, phosphatidylethanolamine, MVB-specific LBPA, and GPI coupled proteins such as CD55 and CD59. Other common constituents of exosomes are tetraspanins (CD81, CD63, CD9), heat shock proteins (HSP60, HSP70, HSP90), and MHC I/II antigens. Annexins regulate cytoskeleton and cell membrane fusion, Rab proteins control the exosome secretion pathway, and GTPases favor coupling and fusion between membranes and ESCRT proteins [19,20] (Figure 2).
Figure 2. Exosome molecular cargo: Depending on their cellular origin (immune, cancer, epithelial, or mesenchymal cells), exosomes may have diverse molecular cargos which may include immunomodulatory proteins involved in vesicle trafficking and enzymes with functional activities in target cells. In the lumen of exosomes, they transport nucleic acids such as DNA, mRNA, miRNA, lncRNA, and other RNA species. Image created with biorender.com.
In general, exosome protein expression is related to the cell from which they originate but also depends on their physiological status, e.g., exosomes released from both human and murine B-cells transformed by the Epstein-Barr virus secrete antigen-presenting vesicles capable of inducing antigen-specific MHC class II-restricted T-cell responses, suggesting a role for exosomes in antigen presentation in vivo [21]. They play a role in hepatitis B virus transmission and NK-cell dysfunction during chronic infection [22], can favor cancer progression, metastasis, and drug resistance by altering gene expression in surrounding and distant cells [23], and be a source of proteins that could have clinical application as biomarkers for the early detection, diagnosis, prognosis and treatment of diseases [24].
3. Role of Exosomes in Antigen Presentation
Immune system modulation by exosomes can occur between cells of the same or different lineages. Effector cells (T, NK, APC, and mast cells) may give or receive information through receptor-ligand interactions or by extracellular vesicles. Tumor cell-derived exosomes (TEX) can prevent immune activation, DC maturation, T and NK cell-mediated cytotoxicity, or promote immune suppression, tolerance, and T-cell apoptosis because they can carry a large diversity of molecules to mediate intercellular communication, (Figure 1) [25,26].
As the first signal required for the activation of an effective immune response is the recognition of the HLA/peptide complexes by the T-cell receptor, and exosomes released by mature APCs express HLA/peptide complexes and costimulatory molecules necessary for T-cell activation, it seems that mature APC exosomes constitute a presentation route as crucial as that of professional APCs for T-cell activation [27,28]. In murine models, bone marrow DCs (BMDCs) have been shown to secrete exosomes capable of amplifying immune responses in vivo by transporting antigens to induce antigen-specific CD4 and CD8 T-cell activation [29–31]. On the other hand, regulatory activities are also carried out by exosomes. A tolerance mechanism mediated by IL-10 production, with an effect on regulatory T-cells (Treg) related to the accumulation of MFG-E8/lactadherin, has been described on exosomes from immature DCs [32,33].
Clayton et al. reported that human tumor-derived exosomes express ligands for NKG2D and TGFb1 that triggered downregulation of NKG2D surface expression by NK and CD8(+) T-cells as an evasion mechanism to avoid their recognition and immune destruction, suggesting that NKG2D is a physiological target for exosome-mediated immune evasion in cancer [34]. The downregulation of pro-inflammatory cytokines such as IL-12p40, IL-23p19, TNF-α, and IL-1β, has also been described induced by endothelial cell-derived exosomes and the overexpression of immunosuppressive ones such as IL-10, MRC1, and TGF-β, to prevent damage and promote tissue regeneration favoring tumor growth and metastasis [35,36]. On the other hand, DCs pulsed with tumor peptides release exosomes with the ability to stimulate CD8+ T-cell proliferation and differentiation into cytotoxic T-cells. It has been also reported that exosomes can stimulate cytotoxic effects on NK cells by membrane IL-15Rα and NKG2D expression, which induce proliferation and membrane IFN production [37,38] Exosomes derived from DCs, which express major histocompatibility complex (MHC) and costimulatory molecules, have been used for antitumor vaccines because, besides to be a source of tumor peptides, they also express molecules essential for the induction of immune responses, such as MHC I, MHC II and costimulatory CD40, CD54, and CD80 [39].
NKG2D is an activating receptor for CD8+ and T-cells, NK and NKT cells. Its expression can be deregulated by its soluble ligands and by growth factors such as TGFβ1, secreted by the tumor as an evasion mechanism to prevent its recognition and immune destruction. In fact, TGFβ1 and soluble ligands for NKG2D have been detected in cancer cell lines and tumor cells isolated from mesothelioma pleural effusions. Downregulation of NKG2D expression on NK and the CD8 + T-cell surface [34], or the decrease in the secretion of the proinflammatory cytokines induced by these exosomes, prevents damage and promotes tissue regeneration, thus promoting tumor growth and metastasis [35,36].
Tumor-derived exosomes and microvesicles (EMVs) are key mediators shed by cancer cells with the ability to sensitize neighboring cells in the tumor microenvironment, thus promoting cancer invasion and metastasis. EMVs derived from hypoxic tumor cells differ qualitatively from those derived from normoxic tumor cells. Hypoxic EMVs inhibit NK cell function to a greater extent than normoxic ones, by transferring TGF-β1 to NK cells, inhibiting NK cell function and decreasing NKG2D expression on the NK cell surface. They also carry high levels of miR-210 and miR-23a, which act as additional immunosuppressive factors by targeting CD107a expression in NK cells. By releasing extracellular vesicles into the hypoxic tumor microenvironment, tumor cells can educate NK cells to decrease their antitumor immune response [40].
4. Tumor-Derived Exosome-Mediated Immune Suppression
Exosome production by tumor cells has been implicated in cancer-associated immune suppression, and it has been proven that the body fluids of cancer patients contain large numbers of tumor exosomes capable of downregulating the functions of immune cells and promoting tumor progression through various mechanisms including the transport of molecules such as proteins and nucleic acids. There is evidence that miRNAs, secreted by the tumor into exosomes can regulate gene expression in target cells through canonical binding to their target mRNAs. Furthermore, it has been shown that miR-21 and miR-29a secreted by the tumor can function by binding to the Toll-like receptors (TLR) family on immune cells. This binding triggers a prometastatic inflammatory response through the activation of NF-κβ, which favors tumor development and metastasis [41]. Unlike exosomes released by normal cells, tumor exosomes are involved in the regulation of peripheral tolerance. They have the ability to down-regulate CD3ζ and JAK3 expressions in primary activated T-cells, induce Fas/FasL-mediated apoptosis in TCD8+ lymphocytes and promote CD4+CD25− T-cell proliferation and their further conversion to CD4+CD25hi+FOXP3+ Treg cells expressing IL-10, TGFβ1, CTLA-4, GrB/perforin to mediate immunosuppression [42,43].
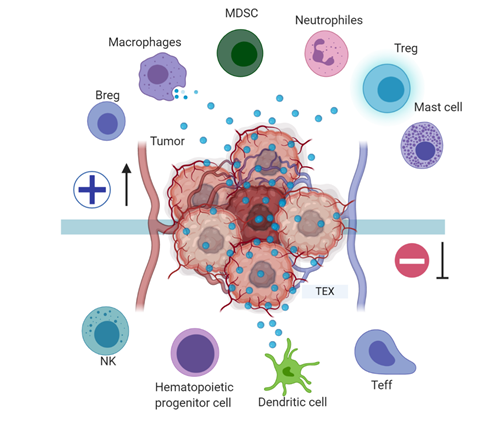
Tumor exosomes, in addition to regulating the immune effector cell (TL, NK, APC) function, are also involved in blocking myeloid cell maturation to DCs through the expression of TGFβ, which induces a CD14+HLA-DR-phenotype, characteristic of myeloid-derived suppressor cells (MDSC). These MDSC include DC precursors with a suppressive effect on proliferation and cytotoxic functions of tumor-specific T-cells by altering antigen processing and presentation, producing inhibitory factors such as nitric oxide and reactive oxygen species [44], and have been described in the peripheral blood of patients with oncological pathologies including hepatocellular carcinoma, bladder carcinoma, glioblastoma and multiple myeloma [45] (Figure 3). A murine model of breast cancer showed that tumor exosome induction of IL-6 expression can block bone marrow DC differentiation, and that pancreatic cancer-derived exosomes can regulate the expression of TLR4 and cytokines, such as TNFα and IL-12 expression in DCs through miR-203 [46,47].
Figure 3. Tumor immune escape mediated by tumor exosomes: Tumor exosomes favor immune escape by bearing proteins such as TGF-β, FasL, TRAIL, PD-L1, and HSP90, and miRNA such as miR-23a, miR-24-3p, and miR-214, to dysregulate NK, T, and B effector cells function. In dendritic cells, exosome miR-203 may downregulate TLR4 expression and increase IL-6 production, inhibiting the differentiation of myeloid precursors into DCs. TEX bearing miR-21, miR-29a, and proteins such as CSF-1, CCL2, and TGFβ may drive macrophage polarization to an M2-like phenotype and promote MDSCs differentiation contributing to cancer progression. Image created with biorender.com.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13040847