Glycolysis is a crucial metabolic process in rapidly proliferating cells such as cancer cells. Phosphofructokinase-1 (PFK-1) is a key rate-limiting enzyme of glycolysis. Its efficiency is allosterically regulated by numerous substances occurring in the cytoplasm. However, the most potent regulator of PFK-1 is fructose-2,6-bisphosphate (F-2,6-BP), the level of which is strongly associated with 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase activity (PFK-2/FBPase-2, PFKFB). PFK-2/FBPase-2 is a bifunctional enzyme responsible for F-2,6-BP synthesis and degradation. Four isozymes of PFKFB (PFKFB1, PFKFB2, PFKFB3, and PFKFB4) have been identified. Alterations in the levels of all PFK-2/FBPase-2 isozymes have been reported in different diseases.
- PFKFB3
- PFKFB4
- PFK-2
- 6-phosphofructo-2-kinase/fructose-2
- 6-bisphosphatase
- 3PO
- PFK-158
- PFK-15
- autophagy
- angiogenesis
- cancer
1. Introduction
Glycolysis is an essential enzymatic process in human cell metabolism. It participates in the production of substrates that are required in multiple biochemical pathways, such as the tricarboxylic (TCA) acid cycle, pentose phosphate pathway (PPP), and fatty acids and cholesterol synthesis. In normal human cells (with the exception of red blood cells), anaerobic reactions predominate in the metabolism under reduced oxygen conditions. However, in 1927, Otto Warburg reported an essential role of glycolysis in cancer cells regardless of oxygen concentration in the tumor microenvironment [1,2,3]. This reprogramming of cancer cell metabolism is not only responsible for its aggressive growth but may also cause a beneficial decrease in Reactive Oxygen Species (ROS) generation and key metabolites for cell growth [4]. It is worth noticing that a similar shift in metabolism is found in proliferative normal cells such as lymphocytes and endothelial cells in angiogenesis [5]. In recent years, targeting key regulatory steps of glycolysis has increasingly become an area of interest among scientists. There are many reports on novel inhibitors affecting distinct molecular targets in this process [6]. Amino acid sequence alterations leading to changes in enzyme catalytic activity have been detected in numerous proteins involved in glycolysis in different types of cancer [7].
Glycolysis intensity is regulated by the activity of three physiologically irreversible enzymes: hexokinase, phosphofructokinase-1 (PFK-1), and pyruvate kinase. PFK-1 is the main rate-limiting enzyme of glycolysis and is responsible for the synthesis of fructose-1,6-bisphosphate from fructose-6-phosphate (F-6-P). Its activity is regulated by cytoplasmically localized metabolic products, such as adenosine triphosphate (ATP), adenosine diphosphate (ADP), F-6-P, and fructose-2,6-bisphosphate (F-2,6-BP) (Figure 1) [8]. Of these compounds, F-2,6-BP, a product of the reaction catalyzed by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK-2/FBPase-2, PFKFB), is the most potent positive allosteric effector of PFK-1 [9]. PFK-2/FBPase-2 is a bifunctional enzyme responsible for the catalyzation of both the synthesis and degradation of F-2,6-BP mediated through its N-terminal domain (2-Kase) and C-terminal domain (2-Pase), respectively [10]. Of note, the active site of the 2-Kase domain has two distinct areas (the F-6-P binding loop and ATP-binding loop) essential for its function [4].
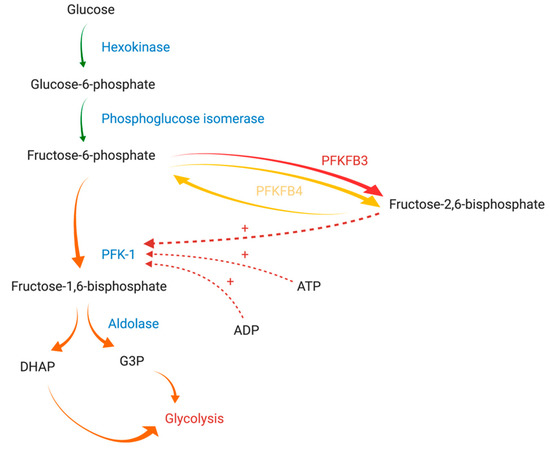
In humans, PFK-2/FBPase-2 is encoded by four different genes: PFKFB1, PFKFB2, PFKFB3, and PFKFB4 [13]. Thus far, four different PFK-2/FBPase-2 isozymes (PFKFB1, PFKFB2, PFKFB3, and PFKFB4) have been identified. Isozymes are characterized by tissue and functional specificity [14]. PFKFB1 can be found in the liver and skeletal muscle, PFKFB2 predominates in cardiac muscle, PFKFB3 is ubiquitously expressed, while PFKFB4 occurs mainly in testes [11]. The overexpression of two isozymes (PFKFB3 and PFKFB4) has been demonstrated in various solid tumors and hematological cancer cells [15,16,17].
Furthermore, due to slight differences in amino acid sequences at key sites for enzymatic activity, all of the isozymes have a different affinity for the synthesis or degradation of F-2,6-BP. Their activity is expressed as the kinase/phosphatase ratio (also termed the 2-Kase/2-Pase activity ratio) [11]. This ratio is about 4.6/1 for PFKFB4 and 730/1 for PFKFB3, while it does not exceed 2.5/1 for PFKFB1 and PFKFB2. Isoforms commonly expressed in tumors satisfy increased energetic requirements of neoplastic cells more efficaciously. Thus, glycolysis, the hallmark of malignancy, might be vulnerable to the therapy affecting only isoforms characterized by a high kinase/phosphatase ratio [10,18].
2. PFKFB3 and PFKFB4 in Cancer
PFK-2/FBPase-2 family members, PFKFB3 and PFKFB4 in particular, are overexpressed in numerous malignancies. PFKFB3 is frequently found in breast cancer [35,59,64,65], colon cancer [35], nasopharyngeal carcinoma [66], pancreatic cancer [67], gastric cancer [67], and many other neoplasms. Similarly, increased transcription of PFKFB4 is observed in pancreatic cancer [67], gastric cancer [67], ovarian cancer [68], breast cancer [35,69], colon cancer [35,70] and glioblastoma [71]. The significance of PFKFB3 level has been reported in cancer cells but also in tumor-related cells such as cancer stem cells. Furthermore, lower PFKFB3 and PFK-I expression levels have been demonstrated in induced pluripotent stem (iPS) cells compared to cancer and cancer stem cells (CSCs). This distinct expression pattern of PFKFB3 may improve the timely detection of CSCs [64].
Influence of PFKFB3 and PFKFB4 on Carcinogenesis
PFKFB3 and PFKFB4 affect carcinogenesis and cancer metabolism in a multidirectional manner. Both isozymes participate in the regulation of glucose metabolism through enhancing glycolysis and PPP. These enzymatic reactions are crucial for cancer development [11]. Increased glucose metabolism through glycolysis enables cancer cells to survive in a microenvironment with limited oxygen supply and produce lactate which acidifies the adherent tissues and thus accelerates metastatic development. On the other hand, redirection of glucose to PPP allows for the synthesis of lipids and nucleic acids essential for the growth of cancer cells. The expression of both enzymes is induced by hypoxia, thereby facilitating nonoxidative glucose-dependent energetic metabolism of the cell. PFKFB3 and PFKFB4 stimulate glucose uptake and boost glycolytic flux to cancer cells by increasing F-2,6-BP, which is a compound promoting glucose utilization by glycolysis [8]. Both proteins are directly engaged in the production of ATP and Nicotinamide adenine dinucleotide (NADH), the synthesis of nucleic acids, and thus cancer cell growth.
3. Targeting PFK-2 Isozymes in Malignancies
3.1. Outline of the Development of Inhibitors
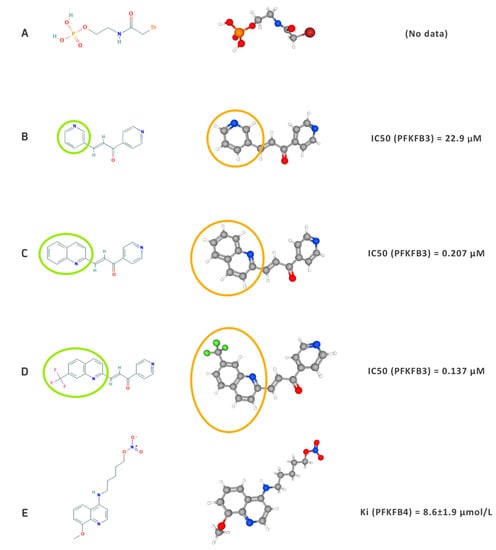
3.2. Chemosensitivity, Chemoresistance, and Potential Combined Therapies for Malignancies
Current chemotherapeutic and irradiation protocols target rapidly dividing cells. Targeting glycolysis, a process that is regulated by PFKFB and is crucial for ATP generation in proliferating cancer cells seems to be a promising therapeutic approach with anticancer properties. Combining currently used chemotherapeutics (conventional or tumor pathway-specific agents) with PFKFB3 or PFKFB4 inhibitors is expected to enrich the range of treatment options. Despite the exponential development of new drugs, the occurrence of resistance simultaneously increases as well; this emphasizes the importance of implementing drug combinations or adding novel therapeutic agents in an attempt to overcome this hurdle [160]. PFKFB inhibition might prevent disease progression and drug resistance, and even improve progression-free survival (PFS) and/or response rates [10,32]. Another argument in favor of combination therapy with these inhibitors is the association between drug resistance, mitochondrial respiratory defects, and increased glycolysis in cancer cells [11,32,161]. Multiple trials verifying currently approved agents, such as inhibitors of angiogenesis or autophagy, and substances interfering with the electron transport chain or glutamine metabolism, in combination with PFKFB inhibitors are expected to be initiated in cohorts with distinct types of cancers [83]. Liu et al. (2001) suggested that the inhibition of glycolysis markedly sensitizes slow-growing cancers to chemotherapy and irradiation [162].
This entry is adapted from the peer-reviewed paper 10.3390/cancers13040909
