Obesity is a challenging condition of excess body fat, caused by an imbalance in energy consumption and expenditure.
- anti-obesity
- phytochemicals
- energy balance
- gut microbiota
1. Introduction
Obesity is a challenging condition of excess body fat, caused by an imbalance in energy consumption and expenditure [1]. It is the result of a complex interaction between environment, diet, genetics, lifestyle, endocrine disorders, medication, and psychological factors [2]. As per the World Health Organization (WHO), individuals with a body mass index (BMI) of more than 30.0 kg/m2 are considered obese, and those with BMI between 25.0 and 30.0 kg/m2 are categorized as overweight [3].
Obesity is associated with commodities like diabetes, hypertension, hyperlipidemia, cancer, and sleep apnea, and is considered as an independent risk factor for cardiovascular diseases (CVD) [4]. An increase by one unit BMI causes a 4% rise in ischemic risk and a 6% rise in hemorrhagic strokes [5]. Higher systolic and diastolic blood pressure of 3.0 mm and 2.3 mm Hg, respectively, in individuals with 10 kg excess body weight can induce a 12% increased risk of CVD and 24% increased risk of stroke [6]. Besides, obesity is also associated with an increased risk of several cancers, including colon, endometrial, kidney, esophageal, liver, pancreatic, breast, Hodgkin’s lymphoma, and myeloma [7,8]. According to the WHO, the incidence of obesity has tripled since 1975. In a survey conducted in 2016, 1.9 billion adults and 340 million children and adolescents were reported to be obese or overweight [9]. The rapid increase in obesity prevalence and its associated devastating health effects and comorbidities highlight the immediate need for early recognition, control, and treatment of this problem. Although diet control, exercise, and lifestyle changes are the fundamental therapeutic options, few drugs have been approved for pharmacotherapy. Several natural products have been widely studied and numerous review articles reference the use of herbs for weight management [10,11,12].
2. Pharmaceutical Drugs for Obesity
Several anti-obesity drugs have been evaluated since the beginning of the 20th century. The earliest drugs were the thyroid hormones due to their thermogenic effect, followed by chemicals such as dinitrophenol, mitochondrial uncouplers, amphetamines, and serotonergic as appetite suppressors, which were subsequently withdrawn due to safety concerns [13]. Following this, poly diet pills using the combination of amphetamines and thyroid hormones along with several other ingredients were distributed as rainbow pills for diet control. Though they were highly popular, fatalities associated with their indiscriminate use led to their withdrawal mandated by the US FDA [13]. A list of drugs and their present status is presented in Table 1. The search for novel drugs to reduce appetite and improve glucose metabolism to control obesity and diabetes is being pursued with renewed vigor and attention as the economic burden associated with obesity exceeds US$200 billion in the United States alone and is increasing steadily [14].
Table 1. List of pharmaceutical drugs and their present status. (Adapted from [15,16,17]).
| Drug Name | Mechanism of Action | Contraindications and Side Effects | Year Approved and Present Status |
|---|---|---|---|
| Phentermine | Centrally acting sympathomimetic agent, appetite suppressant | Increased blood pressure and heart rate. | 1959 Approved for short term |
| Fenfluramine | Increasing serotonin levels through decreasing reuptake of serotonin | Heart valve damage and major adverse cardiovascular events |
(1973–1997) Withdrawn |
| Dexfenfluramine | (1996–1997) Withdrawn | ||
| Sibutramine | (2001–2002) Withdrawn | ||
| Orlistat | Inhibiting pancreatic lipase | Hypertension, Diabetes, Hyperlipidemia | 1999 Approved |
| Rimonabant | Selective central cannabinoid (CB1) receptor antagonist | Psychiatric adverse events | (2006–2007) Withdrawn |
| Lorcaserin | Selective serotonin 2c (5-HT2c) receptor agonist | Occurrence of cancer | (2012–2020) Withdrawn |
| Phentermine-topiramate | Slowing gastric motility and suppressing appetite | Glaucoma, Hyperthyroidism | 2012 Approved |
| Liraglutide (saxenda) | GLP-1 receptor agonist control appetite by mimicking the natural hormone | Medullary thyroid cancer, multiple endocrine neoplasia type 2, C-cell hyperplasia of thyroid, decreased kidney function and pancreatitis | 2014 Approved |
| Semaglutide | Phase III |
3. Pathophysiology of Obesity
In the last few decades, significant advances have been made in understanding the pathophysiological mechanisms involved in obesity. The neuroendocrinal feedback associated with pathological overeating coupled with physical inactivity seems to be the major factors governing obesity. Apart from this, genetic predisposition, hormonal imbalance, and gut microbial dysbiosis also contribute to the accumulation of fat stores [18]. Figure 1 describes the pathophysiological parameters causing obesity.
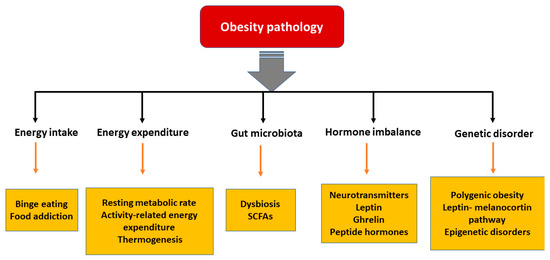
Figure 1. Various parameters affecting the pathology of obesity. [SCFA: short chain fatty acids].
3.1. Energy Intake vs. Expenditure
Obesity can be viewed as an imbalance in energy intake versus energy expenditure. Three components involved in energy expenditure are (i) resting metabolic rate, which is the energy necessary to fuel the body at rest, (ii) activity-related energy expenditure and (iii) diet-induced thermogenesis, which is the energy spent in absorbing and metabolizing food consumed [19]. When an individual ingests more energy than their expenditure, a positive energy balance develops, and this excess energy is converted into triglycerides and stored. When energy intake exceeds energy expenditure by more than 20 kcal/day, 1 kg of fat per year gets accumulated [20]. Thus, a proper balance of energy intake and expenditure is necessary to manage obesity. A complex physiological control system involving signals from the periphery about the status of stored energy, and those that affect energy intake and expenditure, are responsible for maintaining the energy balance [21].
Energy Expenditure and Thermogenesis
The adipose tissue is generally considered as a passive depot for the storage of excess calories. Recent studies have shown that it is an active endocrine organ, which takes part in energy balance by releasing free fatty acids, proinflammatory cytokines, and adipokines such as leptin and adiponectin, regulating food intake and insulin sensitivity [22]. Adipose tissues are heterogeneous and are classified into white adipose tissue (WAT), which are storage organs, and brown adipose tissue (BAT), which burns energy for thermogenesis. Intermediary beige adipocytes, arising from WAT, have also been described in mammals [23]. Exposure to cold, adrenergic stimulation and long-term treatment with peroxisome proliferator-activated receptor (PPAR)γ agonists are some of the external cues that induce these beige adipocytes [24]. The brown and the beige adipocytes contain numerous mitochondria and express the uncoupling protein 1 (UCP-1), which regulates energy expenditure, reduces adiposity, and protects experimental animals from diet-induced obesity [25]. Besides UCP-1, brown adipocytes express type 2 iodothyronine deiodinase (DIO2), the transcription coregulators PR domain containing 16, (PRDM16) and Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), and Cell death activator (CIDE-A), which regulates UCP1 transcription in BAT [26]. With the understanding that BAT can help in energy dissipation, pharmacological interventions targeting browning of WAT are being actively investigated to induce energy balance. Few natural ingredients have been reported to convert WAT to BAT, but Forskolin, Capsaicin, Resveratrol, and Berberine are examples of molecules that could induce browning of WAT [27,28].
3.2. Hormonal Imbalance
Neurobiological mechanisms are reported to contribute to eating in the absence of energy demand and hunger. The cortico-limbic system, hypothalamus and hindbrain are three heavily interconnected brain regions that are involved in controlling eating behavior [29]. These are affected by various visual food stimuli under conditions of fasting, weight loss, overfeeding, exercise, hormone infusion, leanness, obesity, and voluntary cognitive control [30]. The important neurostimulators such as serotonin and dopamine play a significant role in food intake. Increased serotonergic signaling is associated with decreased food intake, whereas its decrease induces hyperphagia and weight gain [31]. Likewise, lesser dopaminergic signaling promotes overconsumption of food beyond homeostatic needs [32].
The gut hormones play the most critical role in obesity. The cells of the gastrointestinal tract sense ingested food and release the gut hormones that regulate energy and glucose homeostasis through autocrine, paracrine, and endocrine pathways. Glucagon-like peptide 1 (GLP-1), peptide (PYY) and oxyntomodulin signal the availability of nutrients to the brain and suppress appetite [33]. Additionally, leptin secreted by the adipose tissue acts as a signal for energy availability and promotes satiety. In the absence of food, ghrelin is secreted, which sensitizes the brain to the intake of food [34].
Leptin Resistance
Leptin is an adipokine that regulates food intake, energy expenditure, immune function, and numerous other physiological activities [35]. Circulating leptin concentrations directly reflect the adipose tissue energy stores and it generally enables energy expenditure while reducing food craving [36]. Leptin binds to its receptor in the brain and mediates its action through the neuroendocrine axes. It also attenuates the hyperglycemia caused by insulin deficiency [37]. Since leptin acts as a messenger for peripheral energy stores, increasing its circulating levels was thought to be a potential treatment for obesity [38]. During the progression of obesity, leptin signaling is affected, leading to leptin resistance. In these cases, the leptin levels are high in serum, but it is unable to bind to its receptor and mediate the physiological activity [39]. Obesity is also associated with leptin resistance, which affects leptin signaling and its downstream physiological effects. Obese patients develop leptin resistance despite high circulating levels of adipokine, rendering the leptin therapy ineffective. Alleviating leptin resistance is an exciting research area as potential anti-obesity therapy as no drugs are known for this function (Figure 2).
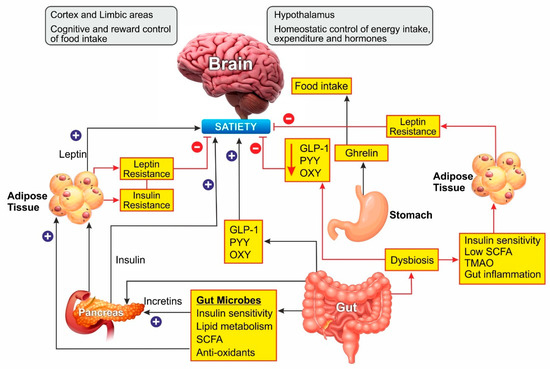
Figure 2. Schematic representation of Gut microbiome and hormonal control of obesity: Ghrelin, produced in the stomach, is a potent stimulator of appetite in the brain. Insulin from the pancreas and leptin from adipose tissue act on the brain to induce satiety. Gut-derived peptides such as GLP-1 augment insulin release from the pancreas. The gut microbiome balances energy homeostasis by releasing short-chain fatty acids with numerous benefits. Dysbiosis of the gut decreases the peptide hormone and increases gut inflammation, which can induce insulin and leptin resistance preventing satiety. In the brain, the hypothalamus region controls energy homeostasis while the cortex and limbal areas are responsible for cognitive reward control of food intake. CCK, cholecystokinin; GLP-1, glucagon-like peptide–1; OXY, oxyntomodulin; PYY, peptide YY; SCFA, short-chain fatty acids. Blue lines represent positive stimulation to control fat storage while red lines represent the causes of obesity.
3.3. Gut Microbiota
Various bacteria, viruses, fungi, and protozoa colonize the gastrointestinal tract, which is interactively involved in immune, metabolic, and neurological health [40,41]. The gut microbes play a major role in metabolism by fermenting the non-digestible dietary fibers to short-chain fatty acids (SCFA) such as butyrate, propionate, and acetate [42]. These SCFAs are involved in cholesterol metabolism and lipogenesis and are reported to play a central role in appetite regulation [43]. In the last two decades, several studies have shown that probiotic supplements can reduce body weight and improve glucose metabolism in rodents by changing the composition of gut microbiota [44]. Overweight and obese people show a dysbiosis characterized by lower microbial diversity associated with impaired glucose homeostasis and low-grade inflammation [45,46]. A meta-analysis of human clinical trials concluded that obesity was associated with higher counts of Firmicutes, Fusobacteria, Proteobacteria, and Lactobacillus reuteri, and lower counts of Bacteroidetes, Akkermansia muciniphila, Faecalibacterium prausnitzii, Lactobacillus plantarum, and Lactobacillus paracasei. An increase in the Firmicutes/Bacteroidetes ratio was observed in association with obesity [47], while a reduction in Firmicutes’ proportion with a rise in Bacteroidetes was associated with weight loss [48]. Further, the gut microbiome composition was found to be directly altered by the diet. In animal models, a high-fat diet favored Firmicutes and lowered the Bacteroidetes [49]. The metabolites derived from the fermentation of food by the microbiome play a vital role in regulating host metabolism. The gut bacteria convert bile acid in the intestine to deoxycholic acid and lithocholic acid, which stimulate the secretion of the incretin hormone GLP-1 and insulin, thereby promoting energy expenditure [50]. Dietary choline metabolism is also linked to microbiome composition. Conversion of choline into trimethylamine-N-oxide (TMAO) by microbiome has been associated with atherosclerosis and metabolic disorders [51]. The conversion of choline to the intermediate trimethylamine is mediated by several intestinal-resident bacteria. The SCFAs produced by gut bacteria are involved in insulin signaling associated with fat accumulation, modulate the secretion of GLP-1 and suppress the inflammatory immune response in the gut [45,52,53]. Inflammation and gut permeability are other markers associated with adiposity [54,55]. These two factors are interlinked as an increased permeability allows bacterial metabolites to leak into the circulation causing low-grade inflammation, a characteristic feature of obesity and insulin resistance [56]. The proinflammatory cytokines, in turn, can cause intestinal barrier disruption [57].
3.4. Genetic Predisposition
Mutation in the leptin-melanocortin pathway [58,59], polygenic obesity [60] and epigenetic disorders like Prader-Willi and Temple syndrome [61] play major roles in the pathogenesis of obesity. Some single gene mutations which disrupt the regulatory system of appetite and weight are described in Table 2. The genetic predisposition to obesity has been reviewed by several authors and is beyond the scope of this manuscript.
Table 2. Monogenic mutations linked to obesity.
| Name | Gene | Chromosomal Position | Action | Reference |
|---|---|---|---|---|
| Leptin | LEP | 7q32.1 | Secreted by adipocytes and functions as a satiety signal in the hypothalamus | [62] |
| Leptin receptor | LEPR | 1p31.2 | Functions as a receptor for leptin to mediate its effect | [63] |
| Proopiomelanocortin | POMC | 2p23.2 | Its deficiency results in the absence α MSH which regulates appetite | [64] |
| Melanocortin 4 receptor | MC4R | 18q21.32 | Appetite regulation binds to α MSH | [65] |
| Single-minded Drosophila Homologue-1 | SIM1 | 6q16.3 | Transcriptional factor required for regulating appetite | [66] |
| Neurotrophic Tyrosine Kinase Receptor Type 2 and Brain-Derived Neurotropic factor | NTRK2, BDNF | 9q21.33, 11p14.1 | These neuro-tropins are involved in the regulation of food intake and body weight | [67] |
| SH2B adaptor protein | SH2B1 | 16p11.2 | Positive regulator of leptin sensitivity | [68] |
This entry is adapted from the peer-reviewed paper 10.3390/nu13020510
