Severe mitral valve regurgitation (MR) carries a significant burden both in prognosis and quality of life of patients, as well as on healthcare systems, with high rates of hospitalization for heart failure. While mitral valve surgery constitutes the first-line treatment option for primary MR in suitable patients, surgical treatment for secondary severe MR remains controversial, with a substantial lack of evidence on a survival benefit. In recent decades, percutaneous mitral valve repair has emerged as an alternative treatment for patients deemed not suitable for surgery. Among several devices under development or evaluation, the MitraClip system is the most widespread and is supported by the strongest evidence. While the role of MitraClip in patients with chronic primary MR who are not deemed suitable for surgery is well established, with consistent data showing improvement in both prognosis and quality of life, MitraClip treatment in secondary MR is a rapidly evolving field. Two recent randomized clinical trials generated apparently controversial results but actually provided an interesting pathophysiologic frame that could help discerning patients who will benefit from the procedure versus patients who will not. In this review, we will discuss current treatment options for mitral regurgitation, focusing on percutaneous mitral valve repair with the MitraClip system.
- Mitral Valve Repair
- heart failure
- mitraclip
- mitral regurgitation
1. Mitral Valve Anatomy and Mitral Valve Regurgitation
The mitral valve apparatus comprises four main components: mitral valve leaflets (anterior and posterior), mitral annulus, chordae tendineae and papillary muscles. Normal mitral valve function allows unidirectional blood flow from the left atrium (LA) to the left ventricle (LV) during diastole, avoiding blood regurgitation in the opposite direction during systole. Mitral valve closing relies on a complex balance between the tethering forces of chordae tendineae and mitral annulus and the closing force generated by LV contraction that results in appropriate coaptation and symmetrical apposition of the leaflets. Any anatomical and/or functional abnormality of one or more of these structures can lead to mitral regurgitation[1].
Primary (degenerative) mitral regurgitation is related to anatomical abnormality of valve leaflets and/or chordae tendinae, while secondary (functional) mitral regurgitation occurs in the setting of LV dilation and systolic dysfunction, which compromises the tethering forces through annular dilation and retraction or displacement of papillary muscle/chordae tendinae, as well as the closing forces due to systolic dysfunction/dyssynchrony [1][2][3][4][5].
Different lesions can determine mitral regurgitation through different mechanisms (Table 1).
Table 1. Functional Carpentier classification [6] of mitral regurgitation and commonest underlying etiologies.
| Leaflet Motion | Lesion | Etiology |
| Type I: normal leaflet motion | Annular dilation/distortion Leaflet perforation |
Dilated cardiomyopathy, left atrial dilation, Endocarditis |
| Type II: excess leaflet motion (prolapse/flail) | Chordal elongation/rupture Papillary muscle rupture |
Degenerative valve disease Ischemic cardiomyopathy, trauma, endocarditis |
| Type IIIA: restricted systo-diastolic leaflet motion | Leaflet and/or chordae thickening/retraction, leaflet calcification/fusion, commissural fusion | Rheumatic heart disease, carcinoid heart disease, dilated cardiomyopathy, radiation |
| Type IIIB: restricted systolic leaflet motion | Papillary muscle displacement or chordal tethering | Ischemic or dilated cardiomyopathy |
2. Pathophysiology and Natural History
Structural, functional and hemodynamic consequences of mitral regurgitation are related to the timing in which valve disease develops.
In acute MR there is acute LV and LA volume overload. Since the mitral valve is functionally in parallel with the aortic valve, mitral regurgitation translates to a sudden decrease in LV afterload and forward cardiac output. Sudden volume overload into a non-dilated LA results in an increase in pulmonary venous pressure and then in pulmonary edema.
In chronic MR, there is an initial compensated stage in which eccentric remodeling of LV can preserve an appropriate forward cardiac output by the increase in LV diastolic volume and stroke volume. However, with progressive LV dilation, LV systolic dysfunction eventually occurs, and there is a progressive hemodynamic compromise with reduction of forward cardiac output and progressive increase in pulmonary venous pressure[7][8][9][10].
If not treated, severe MR is associated with poor prognosis irrespective of its etiology, and heart failure (HF) symptoms development, new-onset atrial fibrillation, LV systolic dysfunction and increase in systolic pulmonary artery pressure (sPAP) constitute the main factors associated with worse outcomes[11][12][13][14][15].
3. Mitral Regurgitation Assessment and Grading
Echocardiography is the primary diagnostic exam in the screening, assessment and grading of mitral regurgitation. It allows a careful assessment of all the structures of mitral valve apparatus, as well as LV volumes and function, LA dimensions and hemodynamic parameters such as sPAP and LV filling pressure estimation. Transesophageal echocardiography (TEE) is widespread used to further characterize the mechanism of MR and in the evaluation for surgical repair/percutaneous repair feasibility.
MR grading is based on qualitative, semi-quantitative and quantitative parameters that can be evaluated through different echo modalities (color, continuous wave doppler, pulsed doppler). When feasible, the PISA (proximal isovelocity surface area) method, used to estimate the size of the effective regurgitant orifice area (EROA), is the most recommended tool in MR severity assessment. According to current European Society of Cardiology (ESC) guidelines, an EROA > 40 mm2 and a regurgitant volume (RVol) > 60 mL indicate severe primary mitral regurgitation, while in secondary MR, lower cutoffs are used (EROA > 20 mm2; RVol > 30 mL) due to evidence supporting a negative prognostic impact of secondary MR above those values [16][17][18][19]. The PISA method is based on the assumption of hemispheric symmetry of the velocity distribution proximal to the circular regurgitant lesion, which may not hold for eccentric jets, multiple jets, or complex or elliptical regurgitant orifices. Practically, the geometry of the PISA varies depending on the shape of the orifice and mitral valve leaflets surrounding the orifice. In functional MR, the PISA might look like an ellipsoidal shape, and two separate MR jets originating from the medial and lateral sides of the coaptation line can be observed on 2D echo. When the shape of the flow convergence zone is not a hemisphere, the PISA method may underestimate the degree of functional MR, and in every case the PISA method could not be applied, assessment of all the other parameters is needed. A list of the main echocardiographic parameters is shown in Table 2 [3][12][13][14][15].
| Echocardiographic Parameters | Data/Values Suggestive of Severe MR |
| Qualitative |
|
| · Morphologic assessment | Prolapse/flail, chordae or papillary muscle rupture |
| · Color flow MR Jet | Large central jet or eccentric jet reaching the posterior wall of LA |
| · Flow convergence zone | Large flow convergence |
| · CW signal of MR jet | Dense/triangular |
| Semi-quantitative | |
| · Vena contracta width | ≥ 7mm |
| · Pulmonary vein flow | Systolic flow reversal |
| Quantitative | |
| · EROA | ≥ 40 mm2 (≥20 mm2 in secondary MR) |
| · Regurgitant volume | ≥ 60 ml (≥30 ml in secondary MR) |
| · Regurgitant fraction | ≥50% |
| Additional evaluation | |
| · LV and LA size | Chamber dilation (may not be present in acute MR; in secondary MR may be a consequence of underlying LV dysfunction) |
| · Estimated sPAP | > 50 mmHg |
4. Surgical Treatment of Mitral Regurgitation
In chronic primary MR, medical therapy of hypertension and/or HFrEF is recommended, if indicated. However, valve surgery (repairing when feasible or replacement) [20][21][22] is the preferred treatment for chronic primary MR. Surgery is indicated in symptomatic patients with severe MR and left ventricle ejection fraction (LVEF) > 30% (Class of recommendation, COR I; Level of evidence, LOE B). In symptomatic patients with severe LV dysfunction (LVEF < 30%, LVESD > 55 mm) refractory to medical therapy, surgery could be considered if the surgical risk is low and there are no major comorbidities (mitral valve repair: COR IIa, LOE C; mitral valve replacement: COR IIb, LOE C) [3]. Surgical treatment in asymptomatic patients is indicated if there are signs of LV dilation and dysfunction (LV EF < 60% or LVESD > 45 mm) (COR I, LOE B) or in patients with preserved LV function (LVEF > 60%, LVESD < 45 mm) presenting new-onset atrial fibrillation (AF) or sPAP > 50 mmHg (COR IIa, LOE B) or in patients with preserved LV function (LVEF > 60%, LVESD 40–44 mm) presenting flail leaflet or significant LA dilation (LAVI > 60 mL/m2 in sinus rhythm) when there is high likelihood or durable valve repair (COR IIa, LOE C) [3][23][24].
In chronic secondary MR, medical and device (CRT if indicated) treatment of HFrEF is recommended[25][26][27], as well as treatment of underlying coronary artery disease, if present. Mitral valve surgery (either repair or replacement) failed to demonstrate a survival benefit in this setting [28][29][30] and only improves symptoms, so current guidelines suggest surgical treatment of secondary MR in patients with LVEF > 30% undergoing coronary artery bypass surgery (CABG) (COR I, LOE C) or symptomatic patients with LVEF < 30% with evidence of myocardial viability and an option for revascularization (COR IIa, LOE C). Mitral valve surgery in symptomatic patients without options for revascularization could be evaluated in patients with LVEF > 30% and low surgical risk (COR IIb, LOE C) [3]. As already mentioned before, mitral valve surgery in secondary MR lacks robust evidence derived from randomized clinical trial, since current guidelines’ indications refer to single-center retrospective studies. The newer interest in the field generated by the development of percutaneous mitral valve repair (PMVR) contributed to filling this gap in knowledge. The ongoing MATTERHORN trial will be the first randomized clinical trial directly comparing MV surgery VS MitraClip in the setting of severe secondary MR in surgical high-risk patients. Acute mitral regurgitation treatment is not the subject of this review and will not be discussed. Table 3 resumes current guidelines’ indications for MR treatment.
| Clinical Setting | Indication for Intervention | Intervention |
| Symptomatic chronic primary mitral regurgitation | · LVEF > 30% | Surgery (COR I, LOE B) |
| · LVEF < 30%, LVESD > 55 mm low surgical risk, no major comorbidities |
Repair (COR IIa, LOE C) or Replacement (COR IIb, LOE C) | |
| · LVEF < 30%, LVESD > 55 mm high surgical risk and/or major contraindication for surgery |
Edge to edge TMVR if feasible (COR IIb, LOE C) | |
| Asymptomatic chronic primary mitral regurgitation | · LVEF < 60% and/or LVESD > 45 mm | Surgery (COR I, LOE B) |
| · LVEF > 60% and new onset AF or sPAP > 50 mmHg | Surgery (COR IIa, LOE B) | |
| · LVEF >60% + LVESD 40–44 mm and flail leaflet or severe LA dilation; low surgical risk | Repair if high likelihood of durable repair (COR IIa, LOE C) | |
| Symptomatic chronic secondary mitral regurgitation | · LVEF > 30% undergoing CABG | Surgery (COR I, LOE B) |
| · LVEF > 30%, low surgical risk | Surgery (COR IIb, LOE C) | |
| · LVEF < 30% with myocardial viability and option for revascularization | Surgery (COR IIa, LOE C) | |
| · LVEF < 30%, high surgical risk | Edge to edge TMVR if feasible (COR IIb, LOE C); ventricular assist device or transplantation program (COR IIb, LOE C) |
5. Percutaneous Treatment of Mitral Regurgitation
Percutaneous mitral valve repair consists of less invasive procedures targeting selected patients with symptomatic chronic primary or secondary MR. Among several devices and techniques that are in ongoing development/evaluation, the MitraClip system (Abbott Laboratories, Menlo Park, CA, USA) is the most widespread and the one with the most robust evidence.
MitraClip is a percutaneous mitral valve repair system that imitates the surgical Alfieri technique, which connects the middle segments of the mitral leaflets through surgical stitches in order to create a double valve orifice and reduce mitral regurgitation[31].
The MitraClip procedure is performed in the catheterization laboratory using echocardiographic and fluoroscopic guidance (Figure 1). The patient is under general anesthesia and systemic anticoagulation with an activated clotting time (ACT) target > 250 s administered. The MitraClip itself is a cobalt chromium clip covered with a polypropylene fabric whose function is to grasp and approximate two opposite segments of the anterior and posterior leaflet. The clip is delivered percutaneously through a venous femoral access. Transseptal puncture is performed in order to introduce the clip delivery system into the left atrium and then into the left ventricle. Once in the left ventricle, the delivery system is steered and aligned over the origin of the regurgitant jet, and leaflet grasping is performed. In its most recent version, the MitraClip system allows for independent leaflets grasping. Once adequate grasping is obtained, desired position of the clip is confirmed and functional assessment of the mitral valve is done, the clip can be released from the delivery system or reopened and repositioned. Residual MR can be targeted by positioning additional clips[32]. Procedural success is defined as proper placement of the device without procedural mortality and with reduction in post-procedural MR by ≥1 grade from baseline and to an absolute level of ≤moderate MR.
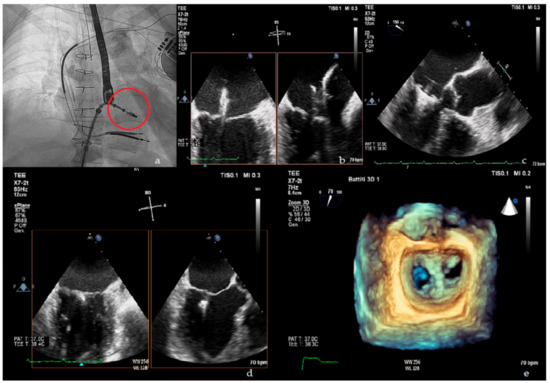
Figure 1. Upper panel: (a) fluoroscopic view of MitraClip delivery system (red circle); (b) X plane transoesophageal echocardiogram (TOE) view of MitraClip in left atrium; (c) TOE left ventricle outflow tract (LVOT) view of MitraClip positioning. Lower panel: (d) X plane TOE view of final clip release; (e) 3D en face view showing the double orifice shape of the mitral valve after clip implantation.
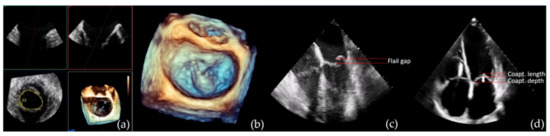
MitraClip suitability and contraindications: Eligibility for MitraClip procedure relies on the analysis of specific anatomical criteria that includes evaluation of leaflets morphology and calcification, planimetric MV area, coaptation length and depth and flail gap and width (Figure 2). Table 4 resumes the optimal suitability criteria as defined in the EVEREST trial[33], as well as suboptimal valve morphology criteria in which MitraClip procedure can still be performed, however with lower success rates [34][35].
| Parameters | Optimal Suitability | Suboptimal/Conditional Suitability |
| Pathology location | A2-P2 | A1-P1 or A3-P3 |
| Calcification | Absent | Mild calcification, not in grasping zone, annular calcification |
| Leaflet mobility | Normal | Systolic restriction |
| Mitral valve area | ≥4 cm2 | ≥3 cm2 |
| Coaptation depth † | <11 mm | ≥11 mm |
| Coaptation length † | ≥2 mm | <2 mm |
| Mobile length of PML | ≥10 mm | 7–10 mm |
| Flail width | ≤15 mm | >15 mm with large annulus size and with the possibility of multiple clip positioning |
| Flail gap | <10 mm |
Procedure contraindications are unfavorable anatomy, intolerance to procedural anticoagulation or post-procedural antiplatelet therapy, active endocarditis, rheumatic MV disease, mitral stenosis, femoral venous, superior vena cava (SVC) or inferior vena cava (IVC) thrombosis or intracardiac left-sided thrombosis or masses, life expectancy <1 year.
Complications: the main procedural and peri-procedural complications are pericardial effusion/tamponade, thrombus formation, access site bleeding, clip detachment from a single leaflet or device embolization, development of mitral stenosis, acute kidney injury and neurological events[36][37][38]. In the EVEREST II trial, major adverse events rate at 30 days was 15% [36]. In a subsequent meta-analysis also including EVEREST II, the weighted mean rate of major adverse effect at 30 days was 17%[37]. ACCESS-EU, a real-world post-approval study including a high risk, elderly population, mainly affected by secondary MR, demonstrated a 30 days major adverse events rate of 17% [39]. More recent trials on secondary MR, which will be later discussed in this review, demonstrated procedural complication rates of 8.5% and 14.6%[40][41].
6. MitraClip in the Current Practice for Primary MR
7. MitraClip for Secondary MR Treatment
7.1. COAPT Trial (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation)
7.2. MITRA–FR
8. Similarities and Differences between COAPT and MITRA–FR
8.1. Medical and Device Therapy at the Baseline
8.2. Echocardiographic Parameters at Baseline
| Baseline Parameters | COAPT | MITRA–FR |
| Etiology of LV dysfunction Ischemic Non-ischemic |
60.7% 39.3% |
59.6% 40.4% |
| LVEDV | 101 ± 34 mL/m2 | 135 ± 35 mL/m2 |
| LVEF inclusion criteria | >20%, <50% | >15%, <40% |
| Mean LVEF | 31% ± 9% | 33 ± 7% |
| EROA cutoff | >30 mm2 | >20 mm2 |
| Mean EROA | 41 ± 15 mm2 | 31 ± 10 mm2 |
| EROA > 30 mm2 | 86% | 48% |
| Additional criteria | LVESD < 70 mm sPAP < 70 mmHg RV dysfunction < moderate |
8.3. Procedural Outcomes
| Outcome | COAPT | MITRA–FR |
| Post-procedural residual MR ≤2 | 95% | 91% |
| 1 year follow up residual ≥3 MR | 5% | 17% |
| % of patients treated with >1 clip | 64% | 54% |
9. A New Concept in the Evaluation of MR Severity: Proportionate vs. Disproportionate MR
-
Patients whose MR severity is proportionate to the degree of LV dilation and dysfunction (proportionate MR).
-
Patients whose MR severity is unexpectedly more compared to their LV dilation and dysfunction (disproportionate MR).
-
Patients whose MR, despite an EROA > 20 mm2, is unlikely to be severe given the greater degree of LV dilation (moderate MR).
Controversies of the “Disproportionate MR” Framework
10. Conclusions
11. Open Questions
-
Whether proportionate and disproportionate secondary MR represent different stages of the same disease or different clinical entities is unclear.
-
A better understanding of pathophysiology of secondary MR could help identify early markers for disproportionate MR and thus prompt treatment.
-
Potential diagnostic performance improvement with cardiac MRI needs to be evaluated. Cardiac MRI could overcome the already mentioned limitations and potential underestimation of the PISA method in MR severity assessment, but current guidelines on valvular disease, as well as the trials on MR treatment presented in this review, does not include it in the diagnostic/therapeutic workup; thus it is still not known if a performance improvement in severity assessment could translate into better patients selection and/or better outcomes; moreover, recent studies have shown possible prognostic implications of left ventricular scar extension detected with cardiac magnetic resonance imaging in patients with secondary mitral regurgitation[51].
-
Beyond MitraClip: In addition to the MitraClip system, several PMVR systems are currently under investigation. One of these is the Edwards PASCAL system, an edge-to-edge mitral valve repair system that has been shown promising results both in safety and efficacy in the 30 days data of the CLASP study[52], as well as a potential to extend MR treatment to patients who do not fulfill eligibility criteria for MitraClip. Moreover, a further therapeutic approach in the treatment of severe mitral regurgitation is represented by a transcatheter mitral valve replacement (TMVR) treatment; this procedure has emerged as a potential therapy for inoperable or high–surgical risk patients with symptomatic mitral regurgitation. The early feasibility of TMVR has been demonstrated in several prior studies[53], with the Tendyne system (Abbott Structural, Santa Clara, CA, USA) representing the largest experience.
This entry is adapted from the peer-reviewed paper 10.3390/hearts2010007
References
- Dal-Bianco, J.P.; Levine, R.A. Anatomy of the mitral valve apparatus: Role of 2D and 3D echocardiography. Cardiol. Clin. 2013, 31, 151–164, doi:10.1016/j.ccl.2013.03.001.
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; EnriquezSarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011, doi:10.1016/S0140-6736(06)69208-8.
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Munoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791, doi:10.1093/eurheartj/ehx391.
- Sattur, S.; Bates, S.; Movahed, M.R. Prevalence of mitral valve prolapse and associated valvular regurgitations in healthy teenagers undergoing screening echocardiography. Exp. Clin. Cardiol. 2010, 15, e13–e15.
- Mirabel, M.; Iung, B.; Baron, G.; Messika-Zeitoun, D.; Détaint, D.; Vanoverschelde, J.L.; Butchart, E.G.; Ravaud, P.; Vahanian, A. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur. Heart J. 2007, 28, 1358–1365, doi:10.1093/eurheartj/ehm001.
- Carpentier, A. Cardiac valve surgery—The “French correction”. J. Thorac. Cardiovasc. Surg. 1983, 86, 323–337.
- Debonnaire, P.; Al Amri, I.; Leong, D.P.; Joyce, E.; Katsanos, S.; Kamperidis, V.; Schalij, M.J.; Bax, J.J.; Marsan, N.A.; Delgado, V. Leaflet remodelling in functional mitral valve regurgitation: Characteristics, determinants, and relation to regurgitation severity. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 290–299, doi:10.1093/ehjci/jeu216.
- McCutcheon, K.; Manga, P. Left ventricular remodelling in chronic primary mitral regurgitation: Implications for medical therapy. Cardiovasc. J. Afr. 2018, 29, 51–65, doi:10.5830/cvja-2017-009.
- Gaasch, W.H.; Meyer, T.E. Left ventricular response to mitral regurgitation: Implications for management. Circulation 2008, 118, 2298–2303, doi:10.1161/CIRCULATIONAHA.107.755942.
- Enriquez-Sarano, M.; Avierinos, J.F.; Messika-Zeitoun, D.; Detaint, D.; Capps, M.; Nkomo, V.; Scott, C.; Schaff, H.V.; Tajik, A.J. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N. Engl. J. Med. 2005, 352, 875–883, doi:10.1056/NEJMoa041451.
- Tribouilloy, C.M.; Enriquez-Sarano, M.; Schaff, H.V.; Orszulak, T.A.; Bailey, K.R.; Tajik, A.J.; Frye, R.L. Impact of preopera-tive symptoms on survival after surgical correction of organic mitral regurgitation: Rationale for optimizing surgical indi-cations. Circulation 1999, 99, 400–405.
- Le Tourneau, T.; Richardson, M.; Juthier, F.; Modine, T.; Fayad, G.; Polge, A.S.; Ennezat, P.V.; Bauters, C.; Vincentelli, A.; Deklunder, G. Echocardiography predictors and prognostic value of pulmonary artery systolic pressure in chronic organic mitral regurgitation. Heart 2010, 96, 1311–1317, doi:10.1136/hrt.2009.186486.
- Tribouilloy, C.; Grigioni, F.; Avierinos, J.F.; Barbieri, A.; Rusinaru, D.; Szymanski, C.; Ferlito, M.; Tafanelli, L.; Bursi, F.; Trojette, F.; et al. MIDA Investigators. Survival implication of left ventricular end-systolic diameter in mitral regurgitation due to flail leaflets a long-term follow-up multicenter study. J. Am. Coll. Cardiol. 2009, 54, 1961–1968.
- Ma, J.I.; Igata, S.; Strachan, M.; Nishimura, M.; Wong, D.J.; Raisinghani, A.; DeMaria, A.N. Predictive factors for progression of mitral regurgitation in asymptomatic patients with mitral valve prolapse. Am. J. Cardiol. 2019, 123, 1309–1313, doi:10.1016/j.amjcard.2019.01.026.
- Singh, R.G.; Cappucci, R.; Kramer-Fox, R.; Roman, M.J.; Kligfield, P.; Borer, J.S.; Hochreiter, C.; Isom, O.W.; Devereux, R.B. Severe mitral regurgitation due to mitral valve prolapse: Risk factors for development, progression, and need for mitral valve surgery. Am. J. Cardiol. 2000, 85, 193–198, doi:10.1016/S0002-9149(99)00645-1.
- Rossi, A.; Dini, F.L.; Faggiano, P.; Cicoira, M.; Frattini, S.; Simioniuc, A.; Gullace, M.; Ghio, S.; Enriquez-Sarano, M.; Tempo-relli, P.L. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011, 97, 1675–1680, doi:10.1136/hrt.2011.225789.
- Ellis, S.G.; Whitlow, P.L.; Raymond, R.E.; Schneider, J.P. Impact of mitral regurgitation on long-term survival after percuta-neous coronary intervention. Am. J. Cardiol. 2002, 89, 315–318, doi:10.1016/S0002-9149(01)02231-7.
- Grigioni, F.; Enriquez-Sarano, M.; Zehr, K.J.; Bailey, K.R.; Tajik, A.J. Ischemic mitral regurgitation: Long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001, 103, 1759–1764, doi:10.1161/01.CIR.103.13.1759.
- Grigioni, F.; Detaint, D.; Avierinos, J.F.; Scott, C.; Tajik, J.; Enriquez-Sarano, M. Contribution of ischemic mitral regurgita-tion to congestive heart failure after myocardial infarction. J. Am. Coll. Cardiol. 2005, 45, 260–267, doi:10.1016/j.jacc.2004.10.030.
- Badhwar, V.; Peterson, E.D.; Jacobs, J.P.; He, X.; Brennan, J.M.; O’Brien, S.M.; Dokholyan, R.S.; George, K.M.; Bolling, S.F.; Shahian, D.M.; et al. Longitudinal outcome of isolated mitral repair in older patients: Results from 14,604 procedures per-formed from 1991 to 2007. Ann. Thorac. Surg. 2012, 94, 1870–1879.
- Lazam, S.; Vanoverschelde, J.L.; Tribouilloy, C.; Grigioni, F.; Suri, R.M.; Avierinos, J.F.; De Meester, C.; Barbieri, A.; Rusinaru, D.; Russo, A.; et al. Twenty-year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation: Analysis of a large, prospective, multicenter, international registry. Circulation 2017, 135, 410–422, doi:10.1161/CIRCULATIONAHA.116.023340.
- Suri, R.M.; Vanoverschelde, J.L.; Grigioni, F.; Schaff, H.V.; Tribouilloy, C.; Avierinos, J.F.; Barbieri, A.; Pasquet, A.; Huebner, M.; Rusinaru, D.; et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regur-gitation due to flail mitral valve leaflets. JAMA 2013, 310, 609–616, doi:10.1001/jama.2013.8643.
- Rosenhek, R.; Rader, F.; Klaar, U.; Gabriel, H.; Krejc, M.; Kalbeck, D.; Schemper, M.; Maurer, G.; Baumgartner, H. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation 2006, 113, 2238–2244, doi:10.1161/CIRCULATIONAHA.105.599175.
- Enriquez-Sarano, M.; Tajik, A.J.; Schaff, H.V.; Orszulak, T.A.; Bailey, K.R.; Frye, R.L. Echocardiographic prediction of surviv-al after surgical correction of organic mitral regurgitation. Circulation 1994, 90, 830–837, doi:10.1161/01.CIR.90.2.830.
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). De-veloped with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200, doi:10.1093/eurheartj/ehw128.
- van Bommel, R.J.; Marsan, N.A.; Delgado, V.; Borleffs, C.J.W.; van Rijnsoever, E.P.; Schalij, M.J.; Bax, J.J. Cardiac resynchro-nization therapy as a therapeutic option in patients with moderate-severe functional mitral regurgitation and high opera-tive risk. Circulation 2011, 124, 912–919, doi:10.1161/CIRCULATIONAHA.110.009803.
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Packer, M.; Clavell, A.L.; Hayes, D.L.; et al. Cardiac resynchronization in chronic heart failure. N. Engl. J. Med. 2002, 346, 1845–1853, doi:10.1056/NEJMoa013168.
- Michler, R.E.; Smith, P.K.; Parides, M.K.; Ailawadi, G.; Thourani, V.; Moskowitz, A.J.; Acker, M.A.; Hung, J.W.; Chang, H.L.; Perrault, L.P.; et al. Two-year outcomes of surgical treatment of moderate ischemic mitral regurgitation. N. Engl. J. Med. 2016, 374, 1932–1941, doi:10.1056/NEJMoa1602003.
- Acker, M.A.; Parides, M.K.; Perrault, L.P.; Moskowitz, A.J.; Gelijns, A.C.; Voisine, P.; Smith, P.K.; Hung, J.W.; Blackstone, E.H.; Puskas, J.D.; et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N. Engl. J. Med. 2014, 370, 23–32, doi:10.1056/NEJMoa1312808.
- Goldstein, D.; Moskowitz, A.J.; Gelijns, A.C.; Ailawadi, G.; Parides, M.K.; Perrault, L.P.; Hung, J.W.; Voisine, P.; Dagenais, F.; Gillinov, A.M.; et al. Two-year outcomes of surgical treatment of severe ischemic mitralregurgitation. N. Engl. J. Med. 2016, 374, 344–353, doi:10.1056/NEJMoa1512913.
- Suradi, H.S.; Kavinsky, C.J.; Hijazi, Z.M. Percutaneous mitral valve repair: The MitraClip device. Glob. Cardiol. Sci. Pract. 2016, 2016, e201617, doi:10.21542/gcsp.2016.17.
- Sherif, M.A.; Paranskaya, L.; Yuecel, S.; Kische, S.; Thiele, O.; D’Ancona, G.; Neuhausen-Abramkina, A.; Ortak, J.; Ince, H.; Öner, A. MitraClip step by step; how to simplify the procedure. Neth. Heart J. 2017, 25, 125–130, doi:10.1007/s12471-016-0930-7.
- Feldman, T.; Kar, S.; Rinaldi, M.; Fail, P.; Hermiller, J.; Smalling, R.; Whitlow, P.L.; Gray, W.; Low, R.; Herrmann, H.C.; et al. Percutaneous mitral repair with the MitraClip system: Safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J. Am. Coll. Cardiol. 2009, 54, 686–694, doi:10.1016/j.jacc.2009.03.077.
- Attizzani, G.F.; Ohno, Y.; Capodanno, D.; Cannata, S.; Dipasqua, F.; Immé, S.; Mangiafico, S.; Barbanti, M.; Ministeri, M.; Cageggi, A.; et al. Extended use of percutaneous edge-to-edge mitral valve repairbeyond EVEREST (Endovascular Valve Edge-to-Edge Repair) criteria: 30- day and 12-month clinical and echocardiographic outcomes from the GRASP (Getting Reduction of Mitral Insufficiency by Percutaneous Clip Implantation) registry. JACC Cardiovasc. Interv. 2015, 8 Pt A, 74–82, doi:10.1016/j.jcin.2014.07.024.
- Lesevic, H.; Karl, M.; Braun, D.; Barthel, P.; Orban, M.; Pache, J.; Hadamitzky, M.; Mehilli, J.; Stecher, L.; Massberg, S.; et al. Longterm outcomes after MitraClip implantation according to the presence or absence of EVEREST inclusion criteria. Am. J. Cardiol. 2017, 119, 1255–1261, doi:10.1016/j.amjcard.2016.12.027.
- Feldman, T.; Foster, E.; Glower, D.D.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; Engeron, E.; et al. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 2011, 364, 1395–1406, doi:10.1056/NEJMoa1009355.
- Wan, B.; Rahnavardi, M.; Tian, D.H.; Phan, K.; Munkholm-Larsen, S.; Bannon, P.G.; Yan, T.D. A meta-analysis of MitraClip system versus surgery for treatment of severe mitral regurgitation. Ann. Cardiothorac. Surg. 2013, 2, 683–692, doi:10.3978/j.issn.2225-319X.2013.11.02.
- Pope, N.H.; Lim, S.; Ailawadi, G. Late calcific mitral stenosis after MitraClip procedure in a dialysis-dependent patient. Ann. Thorac. Surg. 2013, 95, e113–e114, doi:10.1016/j.athoracsur.2012.10.067.
- Maisano, F.; Franzen, O.; Baldus, S.; Schäfer, U.; Hausleiter, J.; Butter, C.; Ussia, G.P.; Sievert, H.; Richardt, G.; Widder, J.D.; et al. Percutaneous mitral valve interventions in the real world: Early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J. Am. Coll. Cardiol. 2013, 62, 1052–1061, doi:10.1016/j.jacc.2013.02.094.
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Ka-padia, S.R.; et al. Transcatheter mitral-valve repair in patients with heart failure. N. Engl. J. Med. 2018, 379, 2307–2318, doi:10.1056/NEJMoa1806640.
- Obadia, J.F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié; D.; et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306, doi:10.1056/NEJMoa1805374.
- Feldman, T.; Kar, S.; Elmariah, S.; Smart, S.C.; Trento, A.; Siegel, R.J.; Apruzzese, P.; Fail, P.; Rinaldi, M.J.; Smalling, R.W.; et al. Randomized comparison of percutaneous repair and surgery for mitralregurgitation: 5-year results of EVEREST II. J. Am. Coll. Cardiol. 2015, 66, 2844–2854, doi:10.1016/j.jacc.2015.10.018.
- Lim, D.S.; Reynolds, M.R.; Feldman, T.; Kar, S.; Herrmann, H.C.; Wang, A.; Whitlow, P.L.; Gray, W.A.; Grayburn, P.; Mack, M.J.; et al. Improved functional status and quality of life in prohibitive surgical risk patients with degenerative mitral re-gurgitation after transcatheter mitral valve repair. J. Am. Coll. Cardiol. 2014, 64, 182–192, doi:10.1016/j.jacc.2013.10.021.
- Nishimura, R.A.; Bonow, R.O. Percutaneous repair of secondary mitral regurgitation—A tale of two trials. N. Engl. J. Med. 2018, 379, 2374–2376, doi:10.1016/j.jcin.2016.06.028.
- Grayburn, P.A.; Sannino, A.; Packer, M. Proportionate and disproportionate functional mitral regurgitation: A new concep-tual framework that reconciles the results of the MITRA-FR and COAPT trials. JACC Cardiovasc. Imaging 2019, 12, 353–362, doi:10.1016/j.jcmg.2018.11.006.
- Grayburn, P.A.; Carabello, B.; Hung, J.; Gillam, L.D.; Liang, D.; Mack, M.J.; McCarthy, P.M.; Miller, D.C.; Trento, A.; Siegel, R.J. Defining severe secondary mitral regurgitation:emphasizing an integrated approach. J. Am. Coll. Cardiol. 2014, 64, 2792–2801, doi:10.1016/j.jacc.2014.10.016.
- Packer, M.; Grayburn, P.A. New Evidence Supporting a Novel Conceptual Framework for Distinguishing Proportionate and Disproportionate Functional Mitral Regurgitation. JAMA Cardiol. 2020, 5, 469–475, doi:10.1001/jamacardio.2019.5971.
- Messika-Zeitoun, D.; Iung, B.; Armoiry, X.; Trochu, J.N.; Donal, E.; Habib, G.; Brochet, E.; Thibault, H.; Piriou, N.; Cormier, B.; et al. Impact of Mitral Regurgitation Severity and Left Ventricular Remodeling on Outcome After Mitraclip Implanta-tion: Results From the Mitra-FR Trial [2020 Sep 16]. JACC Cardiovasc. Imaging 2020, in press, doi:10.1016/j.jcmg.2020.07.021.
- Grayburn, P.A.; Sannino, A.; Cohen, D.J.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.K.; Rinaldi, M.J.; Kapadia, S.R.; Ra-jagopal, V.; et al. Predictors of Clinical Response to Transcatheter Reduction of Secondary Mitral Regurgitation: The COAPT Trial. J. Am. Coll. Cardiol. 2020, 76, 1007–1014, doi:10.1016/j.jacc.2020.07.010.
- Grayburn, P.A.; Packer, M.; Sannino, A.; Stone, G.W. Disproportionate secondary mitral regurgitation: Myths, misconcep-tions and clinical implications. Heart 2020, doi:10.1136/heartjnl-2020-316992.
- Cavalcante, J.L.; Kusunose, K.; Obuchowski, N.A.; Jellis, C.; Griffin, B.P.; Flamm, S.D.; Kwon, D.H. Prognostic Impact of Is-chemic Mitral Regurgitation Severity and Myocardial Infarct Quantification by Cardiovascular Magnetic Resonance. JACC Cardiovasc. Imaging 2020, 13, 1489–1501.
- Lim, D.S.; Kar, S.; Spargias, K.; Kipperman, R.M.; O’Neill, W.W.; Ng, M.K.; Fam, N.P.; Walters, D.L.; Webb, J.G.; Smith, R.L.; et al. Transcatheter Valve Repair for Patients With Mitral Regurgitation: 30-Day Results of the CLASP Study. JACC Cardio-vasc. Interv. 2019, 12, 1369–1378, doi:10.1016/j.jcin.2019.04.034.
- Sorajja, P.; Moat, N.; Badhwar, V.; Walters, D.; Paone, G.; Bethea, B.; Bae, R.; Dahle, G.; Mumtaz, M.; Grayburn, P.; et al. Initial Feasibility Study of a New Transcatheter Mitral Prosthesis: The First 100 Patients. J. Am. Coll. Cardiol. 2019, 73, 1250–1260, doi:10.1016/j.jacc.2018.12.066.
