Molecular hydrogen has attracted great attention in the medical field as a nonfunctional gas that is safe and effective and attenuates oxidative stress by acting as a radical scavenger for hydroxyl radical (•OH) and peroxynitrite (ONOO-). Molecular hydrogen has been reported as an antioxidant and anti-inflammatory agent to treat several oxidative stress related diseases.
- molecular hydrogen
- inflammation
- neuroprotection
- neurological disorder
- oxidative stress
- antioxidant
1. Introduction
H2 works as a moderate but efficient antioxidant[1][2]. Hydrogen is the world’s most abundant element, accounting for about 75% of the world’s mass. Hydrogen is present in water and in organic as well as inorganic compounds. H2 gas is a colorless, odorless, fuel-intensive diatomic gas. There is less than 1 ppm hydrogen gas in the Earth’s atmosphere[3]. H2 does not react with most compounds, including oxygen gas, at room temperature. H2 gas is only inflammable at temperatures exceeding 537 °C. H2 (4–75%, v/v) is explosive due to the rapid oxidation chain reaction. H2 can be dissolved in water under atmospheric pressure to 0.8 mM (1.6 ppm, w/v)[3].
In recent years, various studies related to H2 have attracted researchers’ attention globally, owing to its protective and therapeutic effects[4][5]. Furthermore, hydrogen has a more significant advantage over other gases used for medical purposes, in terms of its toxicity; hydrogen remains non-toxic up to high concentrations and is even used in diving applications[6][7]. Studies have found that the effects of hydrogen inhalation are not apparent and do not affect blood pressure or other parameters, such as pH and temperature. Thus, in comparison, hydrogen has fewer side effects than other antioxidants, as it only decreases •OH[1][8].
2. Administration Routes of Hydrogen
H2 may be administered or taken into the body via various routes. These routes may be divided into three types: H2 gas inhalation, drinking HW, and HS injection. H2 gas inhalation is the simplest and most commonly used method since the initial reports regarding the use of H2[1]. Inhaled H2 diffuses into the lung alveoli and is transported to the entire body. This procedure can, however, be uncomfortable and even dangerous, since H2 gas is explosive at concentrations above 4% in air[2]. Therefore, the mixed gas concentration of H2 is usually maintained between 1% and 4%. Inhaling H2 gas improves acute conditions such as ischemia–reperfusion injury (IRI) and several organ graft injuries. HRW is safer and more comfortable than H2 gas inhalation. It has been reported that HW ad libitum prevents arteriosclerosis among mice with knockout apolipoprotein E, a model for atherosclerosis that develops spontaneously[9]. Consumption of H2 prevents stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice[10]. Recently, the inhalation of H2 and consuming HW showed different adjustments to signal and gene expression in mice[11]. Although the process is invasive, the neuroprotective efficacy in the brain following IRI intraperitoneal injection of HS has been similar to that of H2 gas inhalation[12]. In the human gastrointestinal tract, H2 is produced by intestinal bacteria and plays a key role in metabolic pathways. It functions as a distinctive antioxidant and prevents cardiovascular disorders[5]. One of the studies showed that gut bacteria plays a role in the progression of neurological disorders. In this regard, patients suffering from various CNS disorders were found to have increased intestinal permeability that creates a passage to harmful metabolites from the intestine to the blood which harmfully affect the CNS[13]. A study showed that oral administration of HW leads to protective effects in rat and mice models of PD[14].These findings demonstrate the potential use of HRW for defense against NDs, as well as the possibility of using HRW to treat acute brain disorders, as shown in Figure 1.
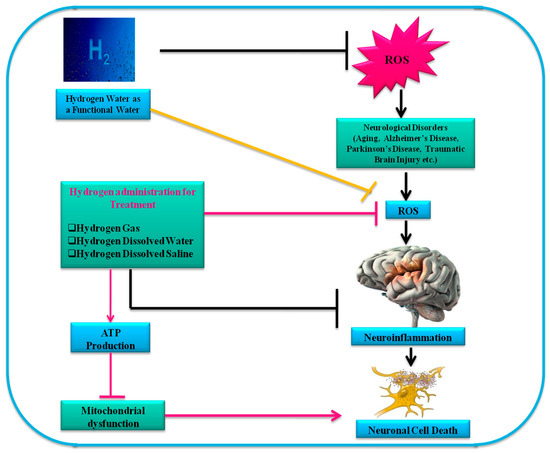
Figure 1. Beneficial effects of H2 on different acute neuronal conditions.
The nuclear factor erythroid 2-related factor 2 (Nrf2) pathway act as a vital role in protecting cells against different stressors and its dysfunction is correlated with decreased tolerance to OS[15]. Nrf2 is an important defense mechanism of the brain against toxins in both, glial and neuronal cells[16][17]. The Nrf2 pathway targets various genes for instance heme oxygenase-1 (HO-1), glutathione S-transferase, SOD, CAT, NAD(P)H dehydrogenase(quinone)1, and others, thus, protecting the neurons of the CNS against OS[18][19]. Nrf2 and various antioxidant enzymes may also increase the expression of anti-inflammatory mediators, phase I and II drug-metabolizing enzymes, and mitochondrial pathways[20][21]. Recent research studies have shown that Nrf2 plays defensive action against the neurotoxins such as 6-hydroxydopamine and 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP), in both, in vitro and in vivo models of PD[22][23]. In this regard, oral administration of HRW has shown a neuroprotective effect against traumatic brain injury (TBI) by activating the Nrf2 signaling pathway. Furthermore, similar findings have been reported in various NDs such as PD, AD, IR, and hemorrhagic stroke, and the effects are attributed to the antioxidant properties of HRW[24][25][26].
3. H2 Acts as an Antioxidant Agent
H2 is highly reactive, protein denaturing, and promotes DNA breakdown. It can selectively reduce •OH and ONOO-, causing a widespread reaction with proteins, lipids, and nucleic acids[27]. Based on animal models and clinical observations, an accumulated body of evidence has shown that H2 can be efficiently used to protect against oxidative damage-associated diseases[28]. It decreases the amount of cytotoxic ROS (•OH), successfully defending cells[28]. Other studies demonstrated similar protective effects of H2 against IRI in organs, such as the liver, heart, and intestines[29]. In rat acute stroke models, 1% to 4% H2 inhalation alleviates infarction dimensions[30]. H2 inhalation prevents critical oxidative damage in 1% to 3% of the cases[31]. HRW intake attenuates learning and memory impairment in mice by reducing oxidative damage. Low-level (1, 3 v/100 v) gas respiration reportedly reduces OS, exceptional hypoxia-induced dyslipidemia, cardiomyocyte hypertrophy, and perivascular fibrosis in left ventricular in C57BL/6J mice[32]. Additionally, the ingestion of HRW triggers the Nrf2/antioxidant protection pathway and antioxidant gene expression to speed up the reduction in oral mucosal impairment in rats[33]. Similarly, the findings of another study showed that HRW had a beneficial effect on acute skin wounds in rats caused by radiation [34]. Several substitute pathways are currently being studied as main components of the energy moderating characteristics of H2, including: (1) ghrelin-linked upregulation of ghrelin receptor (GHS-R1α); (2) ghrelin-linked motivation of glucose transporter 1; (3) non-ghrelin linked stimulation of glucose transporter 4; and (4) non-ghrelin linked improved expression of fibroblast growth factor 21 (FGF21), a regulator of energy expenses[35][36]. HRW has shown neuroprotective properties in a murine MTTP-induced PD model[23][37][38]. Lin and colleagues reported that HRW reduces OS in patients with chronic hepatitis B and metabolic syndrome[39].
Studies have shown that H2 may have benefits by activating the Nrf2 signaling pathway, thus improving antioxidant activity and reducing OS, apoptosis, and inflammation[24][40]. H2 increases the antioxidant activities of enzymes in radiation and TBI through the upregulation of Nrf2[40]. The basic anti-inflammatory mechanism of H2 can even be used by macrophages via the Nrf2 signaling pathway[41]. Nrf2 is a transcription factor that combines antioxidant response elements to control the expression of antioxidants, protecting the body from injury and inflammations against oxidative damages[42].
4. Anti-Inflammatory Effects of H2 in Different Neurodegenerative Disease Models
Numerous studies have reported the anti-inflammatory action of H2[43][41][44]. The rapid spread, high penetrability, and absence of clear side effects are some of its advantages. H2 scavenges ROS radicals and is extremely effective in reducing inflammation in numerous tissues and organs, including the heart, brain, and lungs, and recognized to be a defender against oxidative damage[45][46]. HRW has been widely studied for its ability to inhibit inflammatory reactions and alleviate neuronal apoptosis[47][48]. Microglia is likely to cause neuroinflammation in the brain. Activated microglia and ROS produce pro-inflammatory cytokines. One of the studies showed that H2 has a promising effect on prevention and inflammation related to perinatal brain injury in in vitro and in vivo models[49]. Furthermore, the same study reported that HW prevents lipopolysaccharide (LPS)-induced production of ROS by microglia and reduces LPS-induced microglial neurotoxicity[49]. Several studies have shown that HS can mitigate intestinal infections such as intestinal IR damage, ulcerative colitis, and colon inflammation[50][51]. Moreover, HRW has shown preventive effect against the superoxide ions formation in vitamin C-depleted SMP30/GNL-knockout mice during hypoxia–re-oxygenation conditions[52]. Additionally, one of the studies reported that the addition of H2 to haemodialysis solutions had anti-inflammatory and anti-hypertensive action against the haemodialysis patients, suggesting it as a therapeutic option for uremia patients[53]. In another study, the role of reduction in athletes’ muscle was enhanced by using H2 in the case of intensive physical practice[54]. Domoki and colleagues reported that 2.1% air ventilation augmented by hydrogen substantially maintained cerebrovascular reactivity to hypercapnia and decreased neuronal damage caused by asphyxia-re-ventilation in a perinatal asphyxia newborn pig model[55]. In addition, HRW prevented endoplasmic stress and upregulated HO-1 expression[41]. HRW also ameliorates cognitive impairment in mice with accelerated senescence[31].
5. Effects of Molecular Hydrogen on Animal and Human Models of Neurodegenerative Diseases
PD is caused by the death of dopaminergic neurons at the SNpc of the midbrain and is the second most common ND after AD. PD is caused by two mechanisms: excessive OS and the abnormal ubiquitin–proteasome system[14][56]. Dopamine itself is a prooxidant and dopaminergic cells are intended for exposure to high levels of ROS. In the neuronal cell body, an irregular ubiquitin–proteasome system often induces accumulation of insoluble α-synuclein, resulting in neuronal cell death. By stereotactically injecting catecholaminergic neurotoxin 6-hydroxydopamine into the right striatum, a research group created a rat hemi-PD model, and H2 was shown to have a positive impact[57]. Another study demonstrated a similar prominent effect of HRW on an MPTP-induced mouse model of PD[56]. It is interesting to note that the H2 levels used for MPTP mice were only 5%, the second-lowest in all studies on rodents or humans that had previously been published.
AD is the most common ND and is characterized by irregular β-amyloid (Aβ) and tau accumulation, with large aggregates known as senile plaques and neurofibrillary tangles[58]. Various researches have demonstrated the effects of H2 in different animal models of AD[14][10][24]. One research group reported that administration of HW prevented cognitive impairment and inhibited OS[10]. At the same time, they observed that HW restored neural proliferation of the dentate gyrus after restraint stress[10]. Li and colleagues developed an intra-cerebroventricular injection rat model of Aβ (1–42) AD[59]. With HS treatment, they found that reduced learning and memory impairments and reduced Aβ caused neural inflammation[59]. HS also suppressed lipid peroxidation and inflammatory mediators, such as IL-6 and TNF-α[59]. Furthermore, Wang and colleagues reported that the protective effects of HS may be due to the activation of c-Jun N-terminal Kinase (JNK) and nuclear factor κB (NF-κB) pathways[60]. Additionally, a study in a dementia mouse model reported that administration of HW decreased OS and prevented the decline of memory and cognition while simultaneously increasing the lifespan in the mice. A clinical trial result showed that H2 can notably improve cognition in the apolipoprotein E4 genotype carriers[31]. Studies have shown the relationship of apolipoprotein E in anti-inflammatory, antiapoptotic, and antioxidative effects during brain injuries[61]. In Table 1, the effects of H2 on NDs, such as PD, AD, and other brain conditions are listed.
Table 1. Beneficial effects of H2 against animal and human disease.
| Diseases Category. | Species | Route of Administration | References |
|---|---|---|---|
| Alzheimer’s disease | Animal | Saline | [59][60] |
| Parkinson’s disease | Animal | Water | [56] |
| Corneal alkali-burn | Animal | Instillation | [62] |
| Spinal cord ischemia/reperfusion | Animal | Saline | [63] |
| Surgically induced brain injury | Animal | Gas | [64] |
| Spinal cord | Animal | Saline | [63][65] |
| Spinal cord injury | Animal | Saline | [65] |
| Senile dementia in senescence-accelerated mice | Animal | Water | [10][31] |
| Moderate to severe neonatal brain hypoxia | Animal | Gas | [66] |
| Cerebral infarction | Animal, Human | Gas, saline | [31][67] |
| Glaucoma | Animal | Instillation | [68] |
| Ear, hearing loss | Tissue, Animal | Medium, water | [69][70] |
| Radiation-induced lung injury | Animal | Saline | [71][72] |
| Lung transplantation | Animal | Gas | [73] |
| Burn-induced lung injury | Animal | Saline | [74] |
| Liver ischemia/reperfusion | Animal | Gas | [75] |
| Kidney transplantation | Animal | Water | [76] |
| Diabetes mellitus type I | Animal | Water | [77] |
| Diabetes mellitus type II | Human | Water | [78] |
This entry is adapted from the peer-reviewed paper 10.3390/pr9020308
References
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694.
- Yang, M.; Dong, Y.; He, Q.; Zhu, P.; Zhuang, Q.; Shen, J.; Zhang, X.; Zhao, M. Hydrogen: A Novel Option in Human Disease Treatment. Oxid. Med. Cell Longev. 2020, 2020, 8384742.
- Huang, C.S.; Kawamura, T.; Toyoda, Y.; Nakao, A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic. Res. 2010, 44, 971–982.
- Ohta, S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim. Biophys. Acta 2012, 1820, 586–594.
- Ge, L.; Yang, M.; Yang, N.N.; Yin, X.X.; Song, W.G. Molecular hydrogen: A preventive and therapeutic medical gas for various diseases. Oncotarget 2017, 8, 102653–102673.
- Abraini, J.H.; Gardette-Chauffour, M.C.; Martinez, E.; Rostain, J.C.; Lemaire, C. Psychophysiological reactions in humans during an open sea dive to 500 m with a hydrogen-helium-oxygen mixture. J. Appl. Physiol. 1994, 76, 1113–1118.
- Fontanari, P.; Badier, M.; Guillot, C.; Tomei, C.; Burnet, H.; Gardette, B.; Jammes, Y. Changes in maximal performance of inspiratory and skeletal muscles during and after the 7.1-MPa Hydra 10 record human dive. Eur. J. Appl. Physiol. 2000, 81, 325–328.
- Ono, H.; Nishijima, Y.; Adachi, N.; Sakamoto, M.; Kudo, Y.; Kaneko, K.; Nakao, A.; Imaoka, T. A basic study on molecular hydrogen (H2) inhalation in acute cerebral ischemia patients for safety check with physiological parameters and measurement of blood H2 level. Med. Gas Res. 2012, 2, 1–7.
- Ohsawa, I.; Nishimaki, K.; Yamagata, K.; Ishikawa, M.; Ohta, S. Consumption of hydrogen water prevents atherosclerosis in apolipoprotein E knockout mice. Biochem. Biophys. Res. Commun. 2008, 377, 1195–1198.
- Nagata, K.; Nakashima-Kamimura, N.; Mikami, T.; Ohsawa, I.; Ohta, S. Consumption of molecular hydrogen prevents the stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. Neuropsychopharmacology 2009, 34, 501–508.
- Sobue, S.; Yamai, K.; Ito, M.; Ohno, K.; Ito, M.; Iwamoto, T.; Ichihara, M. Simultaneous oral and inhalational intake of molecular hydrogen additively suppresses signaling pathways in rodents. Mol. Cell. Biochem. 2015, 403, 231–241.
- Liu, Y.; Liu, W.; Sun, X.; Li, R.; Sun, Q.; Cai, J.; Zhang, W. Hydrogen saline offers neuroprotection by reducing oxidative stress in a focal cerebral ischemia-reperfusion rat model. Med. Gas. Res. 2011, 1, 1–9.
- Grochowska, M.; Laskus, T.; Radkowski, M. Gut microbiota in neurological disorders. Arch. Immunol. Ther. Exp. 2019, 67, 375–383.
- Fujita, K.; Nakabeppu, Y.; Noda, M. Therapeutic effects of hydrogen in animal models of Parkinson’s disease. Parkinson Dis. 2011, 2011, 307875.
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1–Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272.
- Katoh, Y.; Itoh, K.; Yoshida, E.; Miyagishi, M.; Fukamizu, A.; Yamamoto, M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells 2001, 6, 857–868.
- Guo, X.; Han, C.; Ma, K.; Xia, Y.; Wan, F.; Yin, S.; Kou, L.; Sun, Y.; Wu, J.; Hu, J.; et al. Hydralazine protects nigrostriatal dopaminergic neurons from MPP+ and MPTP induced neurotoxicity: Roles of Nrf2-ARE signaling pathway. Front. Neurol. 2019, 10, 271.
- Oh, S.; Shimizu, H.; Satoh, T.; Okada, S.; Adachi, S.; Inoue, K.; Eguchi, H.; Yamamoto, M.; Imaki, T.; Hashimoto, K.; et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 2006, 443, 709–712.
- Lim, Y.W. Triple endobutton technique in acromioclavicular joint reduction and reconstruction. Ann. Acad. Med. Singap. 2008, 37, 294.
- Buendia, I.; Michalska, P.; Navarro, E.; Gameiro, I.; Egea, J.; León, R.J. Therapeutics, Nrf2–ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Clin. Pharm. Therap. 2016, 157, 84–104.
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L.J. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 101059.
- Jakel, R.J.; Townsend, J.A.; Kraft, A.D.; Johnson, J.A. Nrf2-mediated protection against 6-hydroxydopamine. Brain Res. 2007, 1144, 192–201.
- Innamorato, N.G.; Jazwa, A.; Rojo, A.I.; García, C.; Fernández-Ruiz, J.; Grochot–Przeczek, A.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Cuadrado, A. Different susceptibility to the Parkinson’s toxin MPTP in mice lacking the redox master regulator Nrf2 or its target gene heme oxygenase-1. PLoS ONE 2010, 5, e11838.
- Wang, F.; Yu, G.; Liu, S.Y.; Li, J.B.; Wang, J.F.; Bo, L.L.; Qian, L.R.; Sun, X.J.; Deng, X.M. Hydrogen-rich saline protects against renal ischemia/reperfusion injury in rats. J. Surg. Res. 2011, 167, e339–e344.
- Huang, L.; Lenahan, C.; Boling, W.; Tang, J.; Zhang, J.H. Molecular hydrogen application in stroke: Bench to bedside. Curr. Pharm. Des. 2021, 27, 1–9.
- Manaenko, A.; Lekic, T.; Ma, Q.; Zhang, J.; Tang, J. Hydrogen inhalation ameliorated mast cell mediated brain injury after ICH in mice. Crit. Care Med. 2013, 41, 1266.
- Yuan, L.; Shen, J. Hydrogen, a potential safeguard for graft-versus-host disease and graft ischemia-reperfusion injury? Clinics 2016, 71, 544–549.
- Liu, Q.; Shen, W.F.; Sun, H.Y.; Fan, D.F.; Nakao, A.; Cai, J.M.; Yan, G.; Zhou, W.P.; Shen, R.X.; Yang, J.M.; et al. Hydrogen-rich saline protects against liver injury in rats with obstructive jaundice. Liver Int. 2010, 30, 958–968.
- Choi, J.; An, E.S.; Ban, Y.H.; Seo, D.W.; Kim, T.S.; Lee, S.P.; Kim, Y.B. Hydrogen-enriched water eliminates fine particles from the lungs and blood by enhancing phagocytic activity. J. Biomed. Res. 2017, 31, 503–511.
- Nishida, T.; Hayashi, T.; Inamoto, T.; Kato, R.; Ibuki, N.; Takahara, K.; Tanda, N. Dual gas treatment with hydrogen and carbon monoxide attenuates oxidative stress and protects from renal ischemia-reperfusion injury. Transplant. Proc. 2018, 1, 250–258.
- Nishimaki, K.; Asada, T.; Ohsawa, I.; Nakajima, E.; Ikejima, C.; Yokota, T.; Ohta, S. Effects of molecular hydrogen assessed by an animal model and a randomized clinical study on mild cognitive impairment. Curr. Alzheimer Res. 2018, 15, 482–492.
- Hayashi, T.; Yoshioka, T.; Hasegawa, K.; Miyamura, M.; Mori, T.; Ukimura, A.; Ishizaka, N. Inhalation of hydrogen gas attenuates left ventricular remodeling induced by intermittent hypoxia in mice. Am. J. Physiol. 2011, 301, H1062–H1069.
- Tamaki, N.; Orihuela-Campos, R.C.; Fukui, M.; Ito, H.O. Hydrogen-rich water intake accelerates oral palatal wound healing via activation of the Nrf2/antioxidant defense pathways in a rat model. Oxid. Med. Cell Longev. 2016, 2016, 5679040.
- Zhou, P.; Lin, B.; Wang, P.; Pan, T.; Wang, S.; Chen, W.; Liu, S. The healing effect of hydrogen-rich water on acute radiation-induced skin injury in rats. J. Radiat. Res. 2019, 60, 17–22.
- Ostojic, S.M. Does H2 alter mitochondrial bioenergetics via GHS-R1α activation? Theranostics 2017, 7, 1330–1332.
- Kamimura, N.; Nishimaki, K.; Ohsawa, I.; Ohta, S. Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity 2011, 19, 1396–1403.
- Yoritaka, A.; Ohtsuka, C.; Maeda, T.; Hirayama, M.; Abe, T.; Watanabe, H.; Hatano, T. Randomized, double-blind, multicenter trial of hydrogen water for Parkinson’s disease. Mov. Disord. Clin. Pract. 2018, 33, 1505–1507.
- Yoritaka, A.; Abe, T.; Ohtsuka, C.; Maeda, T.; Hirayama, M.; Watanabe, H.; Hatano, T. A randomized double-blind multi-center trial of hydrogen water for Parkinson’s disease: Protocol and baseline characteristics. BMC Neurol. 2016, 16, 66.
- Lin, C.P.; Chuang, W.C.; Lu, F.J.; Chen, C.Y. Anti-oxidant and anti-inflammatory effects of hydrogen-rich water alleviate ethanol-induced fatty liver in mice. World J. Gastroenterol. 2017, 23, 4920–4934.
- Kura, B.; Bagchi, A.K.; Singal, P.K.; Barancik, M.; LeBaron, T.W.; Valachova, K.; Šoltés, L.; Slezák, J. Molecular hydrogen: Potential in mitigating oxidative-stress-induced radiation injury. Can. J. Physiol. Pharm. 2018, 97, 287–292.
- Chen, H.G.; Xie, K.L.; Han, H.Z.; Wang, W.N.; Liu, D.Q.; Wang, G.L.; Yu, Y.H. Heme oxygenase-1 mediates the anti-inflammatory effect of molecular hydrogen in LPS-stimulated RAW 264.7 macrophages. Int. J. Surg. 2013, 11, 1060–1066.
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation of Nrf2—An update. Free Radic. Biol. Med. 2014, 66, 36–44.
- Yuan, J.; Wang, D.; Liu, Y.; Chen, X.; Zhang, H.; Shen, F.; Liu, X.; Fu, J. Hydrogen-rich water attenuates oxidative stress in rats with traumatic brain injury via Nrf2 pathway. J. Surg. Res. 2018, 228, 238–246.
- Gao, Y.; Yang, H.; Fan, Y.; Li, L.; Fang, J.; Yang, W. Hydrogen-rich saline attenuates cardiac and hepatic injury in doxorubicin rat model by inhibiting inflammation and apoptosis. Mediat. Inflamm. 2016, 2016, 1320365.
- Tamura, T.; Hayashida, K.; Sano, M.; Onuki, S.; Suzuki, M. Efficacy of inhaled hydrogen on neurological outcome following brain ischemia during post-cardiac arrest care (HYBRID II trial): Study protocol for a randomized controlled trial. Trials 2017, 18, 1–9.
- Haam, S.; Lee, J.G.; Paik, H.C.; Park, M.S.; Lim, B.J. Hydrogen gas inhalation during ex vivo lung perfusion of donor lungs recovered after cardiac death. J. Heart. Lung Transplant. 2018, 37, 1271–1278.
- Ito, M.; Hirayama, M.; Yamai, K.; Goto, S.; Ichihara, M.; Ohno, K.; Ito, M. Drinking hydrogen water and intermittent hydrogen gas exposure, but not lactulose or continuous hydrogen gas exposure, prevent 6-hydorxydopamine-induced Parkinson’s disease in rats. Med. Gas. Res. 2012, 2, 1–7.
- Abisso, T.G.; Adzavon, Y.M.; Zhao, P.; Zhang, X.; Liu, M.; Ma, X. Current progress in molecular hydrogen medication: Protective and therapeutic uses of hydrogen against different disease scenarios. Intern. Med. 2020, 10, 314.
- Imai, K.; Kotani, T.; Tsuda, H.; Mano, Y.; Nakano, T.; Ushida, T.; Hirakawa, A. Neuroprotective potential of molecular hydrogen against perinatal brain injury via suppression of activated microglia. Free Radic. Biol. Med. 2016, 91, 154–163.
- He, J.; Xiong, S.; Zhang, J.; Wang, J.; Sun, A.; Mei, X.; Wang, Q. Protective effects of hydrogen-rich saline on ulcerative colitis rat model. J. Surg. Res. 2013, 185, 174–181.
- Chen, C.H.; Manaenko, A.; Zhan, Y.; Liu, W.W.; Ostrowki, R.P.; Tang, J.; Zhang, J.H. Hydrogen gas reduced acute hyperglycemia-enhanced hemorrhagic transformation in a focal ischemia rat model. Neuroscience 2010, 169, 402–414.
- Sato, Y.; Kajiyama, S.; Amano, A.; Kondo, Y.; Sasaki, T.; Handa, S.; Fujinawa, H. Hydrogen-rich pure water prevents superoxide formation in brain slices of vitamin C-depleted SMP30/GNL knockout mice. Biochem. Biophys. Res. Commun. 2008, 375, 346–350.
- Nakayama, M.; Nakano, H.; Hamada, H.; Itami, N.; Nakazawa, R.; Ito, S.A. Novel bioactive haemodialysis system using dissolved dihydrogen (H2) produced by water electrolysis: A clinical trial. Nephrol. Dial. Transplant. 2010, 25, 3026–3033.
- LeBaron, T.W.; Laher, I.; Kura, B.; Slezak, J. Hydrogen gas: From clinical medicine to an emerging ergogenic molecule for sports athletes. Can. J. Physiol. Pharmacol. 2019, 97, 797–807.
- Domoki, F.; Oláh, O.; Zimmermann, A.; Németh, I.; Tóth-Szűki, V.; Hugyecz, M.; Bari, F. Hydrogen is neuroprotective and preserves cerebrovascular reactivity in asphyxiated newborn pigs. Pediatr. Res. 2010, 68, 387–392.
- Fujita, K.; Seike, T.; Yutsudo, N.; Ohno, M.; Yamada, H.; Yamaguchi, H.; Katafuchi, T. Hydrogen in drinking water reduces dopaminergic neuronal loss in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine mouse model of Parkinson’s disease. PLoS ONE 2009, 4, e7247.
- Fu, Y.; Ito, M.; Fujita, Y.; Ito, M.; Ichihara, M.; Masuda, A.; Ohsawa, I. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson’s disease. Neurosci. Lett. 2009, 453, 81–85.
- Jucker, M.; Walker, L.C. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann. Neurol. 2011, 70, 532–540.
- Li, J.; Wang, C.; Zhang, J.H.; Cai, J.M.; Cao, Y.P.; Sun, X.J. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer’s disease by reduction of oxidative stress. Brain Res. 2010, 1328, 152–161.
- Wang, C.; Li, J.; Liu, Q.; Yang, R.; Zhang, J.H.; Cao, Y.P.; Sun, X.J. Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of JNK and NF-κB activation in a rat model of amyloid-beta-induced Alzheimer’s disease. Neurosci. Lett. 2011, 491, 127–132.
- Noe, E.; Ferri, J.; Colomer, C.; Moliner, B.; Chirivella, J. APOE genotype and verbal memory recovery during and after emergence from post-traumatic amnesia. Brain Inj. 2010, 24, 886–892.
- Kubota, M.; Shimmura, S.; Kubota, S.; Miyashita, H.; Kato, N.; Noda, K.; Ozawa, Y.; Usui, T.; Ishida, S.; Umezawa, K.; et al. Hydrogen and N-acetyl-L-cysteine rescue oxidative stress-induced angiogenesis in a mouse corneal alkali-burn model. Investig. Ophthal. Vis. Sci. 2011, 52, 427–433.
- Chen, C.; Chen, Q.; Mao, Y.; Xu, S.; Xia, C.; Shi, X.; Sun, X. Hydrogen-rich saline protects against spinal cord injury in rats. Neurochem. Res. 2010, 35, 1111–1118.
- Eckermann, J.M.; Chen, W.; Jadhav, V.; Hsu, F.P.; Colohan, A.R.; Tang, J.; Zhang, J.H. Hydrogen is neuroprotective against surgically induced brain injury. Med. Gas. Res. 2011, 1, 7.
- Huang, Y.; Xie, K.; Li, J.; Xu, N.; Gong, G.; Wang, G.; Xiong, L. Beneficial effects of hydrogen gas against spinal cord ischemia–reperfusion injury in rabbits. Brain Res. 2011, 1378, 125–136.
- Matchett, G.A.; Fathali, N.; Hasegawa, Y.; Jadhav, V.; Ostrowski, R.P.; Martin, R.D.; Dorotta, I.R.; Sun, X.; Zhang, J.H. Hydrogen gas is ineffective in moderate and severe neonatal hypoxia–ischemia rat models. Brain Res. 2009, 1259, 90–97.
- Ono, H.; Nishijima, Y.; Adachi, N.; Tachibana, S.; Chitoku, S.; Mukaihara, S.; Nawashiro, H. Improved brain MRI indices in the acute brain stem infarct sites treated with hydroxyl radical scavengers, edaravone and hydrogen, as compared to edaravone alone. A non-controlled study. Med. Gas. Res. 2011, 1, 1–9.
- Oharazawa, H.; Igarashi, T.; Yokota, T.; Fujii, H.; Suzuki, H.; Machide, M.; Ohsawa, I. Protection of the retina by rapid diffusion of hydrogen: Administration of hydrogen-loaded eye drops in retinal ischemia–reperfusion injury. Investig. Ophthalmol. Vis. Sci. 2010, 51, 487–492.
- Taura, A.; Kikkawa, Y.S.; Nakagawa, T.; Ito, J. Hydrogen protects vestibular hair cells from free radicals. Acta. Otolaryngol. 2010, 130, 95–100.
- Lin, Y.; Kashio, A.; Sakamoto, T.; Suzukawa, K.; Kakigi, A.; Yamasoba, T. Hydrogen in drinking water attenuates noise-induced hearing loss in guinea pigs. Neurosci. Lett. 2011, 487, 12–16.
- Terasaki, Y.; Ohsawa, I.; Terasaki, M.; Takahashi, M.; Kunugi, S.; Dedong, K.; Ishikawa, A. Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L415–L426.
- Qian, L.; Cao, F.; Cui, J.; Wang, Y.; Huang, Y.; Chuai, Y.; Cai, J. The potential cardio protective effects of hydrogen in irradiated mice. J. Radiat. Res. 2010, 51, 741–747.
- Kawamura, T.; Wakabayashi, N.; Shigemura, N.; Huang, C.S.; Masutani, K.; Tanaka, Y.; Okumura, M. Inhaled hydrogen gas therapy for prevention of lung transplant-induced ischemia/reperfusion injury in rats. Asthma. Res. Pract. 2010, 90, 1344–1351.
- Fang, Y.; Fu, X.J.; Gu, C.; Xu, P.; Wang, Y.; Yu, W.R.; Yao, M. Hydrogen-rich saline protects against acute lung injury induced by extensive burn in rat model. J. Burn. Care Res. 2011, 32, e82–e91.
- Fukuda, K.I.; Asoh, S.; Ishikawa, M.; Yamamoto, Y.; Ohsawa, I.; Ohta, S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem. Biophys. Res. Commun. 2007, 361, 670–674.
- Cardinal, J.S.; Zhan, J.; Wang, Y.; Sugimoto, R.; Tsung, A.; McCurry, K.R.; Nakao, A. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int. 2010, 77, 101–109.
- Li, Y.; Hamasaki, T.; Nakamichi, N.; Kashiwagi, T.; Komatsu, T.; Ye, J.; Teruya, K.; Abe, M.; Yan, H.; Kinjo, T.; et al. Suppressive effects of electrolyzed reduced water on alloxan-induced apoptosis and type 1 diabetes mellitus. Cytotechnology 2011, 63, 119–131.
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Adachi, T. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143.
