Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
Muscular Dystrophies (MDs) are a group of rare inherited genetic muscular pathologies encompassing a variety of clinical phenotypes, gene mutations and mechanisms of disease. MDs undergo progressive skeletal muscle degeneration causing severe health problems that lead to poor life quality, disability and premature death. There are no available therapies to counteract the causes of these diseases and conventional treatments are administered only to mitigate symptoms. Recent understanding on the pathogenetic mechanisms allowed the development of novel therapeutic strategies based on gene therapy, genome editing CRISPR/Cas9 and drug repurposing approaches. Despite the therapeutic potential of these treatments, once the actives are administered, their instability, susceptibility to degradation and toxicity limit their applications.
- nanoparticles
- Duchenne Muscular Dystrophy
- myotonic dystrophy
- antisense oligonucleotides
- small molecules
- CRISPR/Cas9
1. Introduction
Muscular dystrophies (MDs) are a group of chronic inherited genetic diseases, with a worldwide estimated prevalence of 19.8–25.1 per 100,000 persons [1,2]. These multi-organ diseases mainly affect muscles, especially skeletal muscles, which undergo a progressive degeneration causing severe health problems that lead to poor life quality, loss of independence, disability and premature death [3,4]. Among the various types of MDs described so far, the most commons are the Duchenne Muscular Dystrophy (DMD) and Myotonic Dystrophies (DMs) [2,3,5].
Over the last several years, drug delivery nanosystems, referred to as nanomedicine, have been extensively explored for the development of more effective and safer treatments with main applications in cancers [23,24,25,26], central nervous system-related disorders [27,28,29] and immune diseases [30,31,32]. More recently, nanomedicine has also been investigated for the treatment of viral infections [33] such as the lately approved Moderna’s and Pfizer’s Covid-19 nanoparticle-based vaccines [34,35,36,37]. In cancer therapy, nanomedicine holds potential to improve current treatments by reducing side effects of chemotherapeutic agents. Moreover, combination approaches and immunomodulation strategies have been successfully developed to boost their performances [38,39,40]. Nevertheless, only 15 nanoparticle-based cancer therapies have received clinical approval and entered the market, such as the recent liposomal Onivyde® and Vyxeos® formulations [41,42].
Currently, novel nanomedicines are optimized for the treatment of skeletal muscle pathologies like MDs. However, multiple biological and pharmaceutical barriers challenge nanomedicine delivery to skeletal muscles. Biological barriers are embodied by the complex architecture of the skeletal muscle, which encompasses the skeletal muscle parenchyma itself, connective tissue, blood vessels and nerves. One of the main hurdles for delivery to skeletal muscles lies in the presence of the dense extracellular matrix (ECM), which accounts for 1 to 10% of the muscle mass [43,44,45]. Mostly made of fibrous-forming proteins (collagens, glycoproteins, proteoglycans and glycosaminoglycans) it hampers nanoparticles (NPs) penetration by retaining them in the ECM via electrostatic and mechanical interactions [46,47].
2. New Treatments based on Nanocarriers as Alternative Strategies to Facilitate Skeletal Muscle Targeting
Over the last years, the application of nanomedicine as a promising innovative approach to treat different pathologies such as MDs has been investigated. The architectural and structural complexities of skeletal muscles challenge nanomedicine delivery, especially due to the important presence of ECM [45,140]. To restrict interactions with ECM, administration of NPs by I.V. appears as a potential strategy for targeting skeletal muscle. The dense blood capillary network of skeletal muscles increases NPs access to muscle fibres [141,142]. However, once in the blood circulation, NPs can be rapidly cleared through the mononuclear phagocyte system via opsonisation or complexation with plasma proteins [143,144,145,146]. Physical and chemical instability [147,148], immunogenicity [149,150] or premature degradation [151] are other limiting factors that might interfere with NPs delivery.
In addition, long-term administration is required to cure chronic disorders such as MDs, which makes biocompatibility and biodegradability of the nanosystems important requirements [152]. NPs should persist long enough to reverse muscle damages without involving any additional muscle degeneration, before undergoing gradual degradation [51,153]. Therefore, their design has to be optimised to associate or encapsulate active compounds and to deliver them to skeletal muscles. As illustrated in Figure 3, various NPs structures have been described [154]. RNA- and DNA-based nanocarriers are obtained via electrostatic and hydrophobic-hydrophobic interactions with polymers or lipids [155,156,157]. In the case of delivery of the small molecules, their chemical properties, such as their molecular size, structure and n-octanol-water partition coefficient have an impact on the selection criteria for nanocarrier strategy [158,159]. Interestingly, synthetic nanocarriers interacting by electrostatic and hydrophobic-hydrophobic interactions have been demonstrated to deliver complex CRISPR/Cas9 systems under various forms such as DNA, mRNA or ribonucleoproteins [160,161,162].
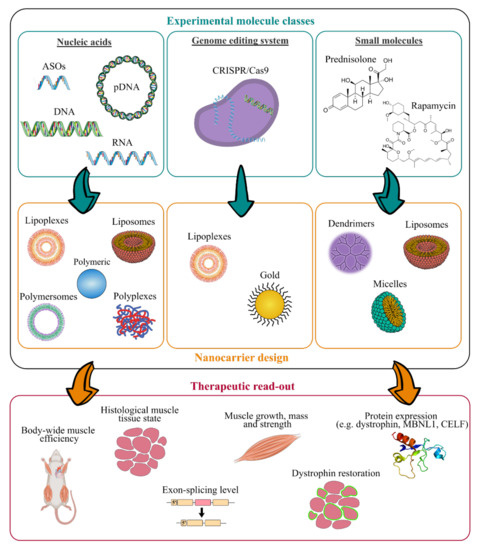
Figure 3. Work flow for the design of innovative nanomedicine and therapeutic readout. (ASOs, antisense oligonucleotides; CRISPR, clustered regularly interspaced short palindromic repeats).
As illustrated in Figure 3, many experimental molecules and macromolecules have been selected as candidates for MD therapies, and a wide range of nanocarriers has allowed their delivery to skeletal muscles, promoting in most of the cases their therapeutic potential.
The present section aims at highlighting nanosystems used for DMD and DM applications that reached preclinical studies. An overview of the various described nanosystems is reported in Table 2.
Table 2. Recapitulative table of the diverse described nanosystems tested in vivo for treating MDs (PEI, polyethylenimine; PEG, polyethylene glycol; PLGA, poly(lactic-co-glycolic acid); PMMA, poly(methyl methacrylate); NIPAM, N-isopropylacrylamide; PEA, poly(ethylene adipate); PLys, poly(l-lysine); PPE-EA, poly(2-aminoethyl propylene phosphate); PAMAM-OH, hydroxyl-terminated poly(amidoamine); DMPC, L-a-dimyristoylphosphatidylcholine; (C12(EO)23), polyoxyethylene(23) lauryl ether; NPs, nanoparticles; ASO, antisense oligonucleotide; PMO, phosphorodiamidate morpholino oligomer; GA, glatiramer acetate; CRISPR, clustered regularly interspaced short palindromic repeats; I.M., intramuscular injection; I.V., intravenous injection; I.P., intraperitoneal).
| Class of Nanocarriers | Nanocarrier Composition | Muscle Pathology | Loaded Molecules |
Therapeutic Target | Mouse Model | Advantages and Limitations | Admin. Route | Ref. |
|---|---|---|---|---|---|---|---|---|
| Polymeric | PEI-PEG | DMD | 2′-OMe ASO | Dystrophin pre-mRNA | mdx | (+) high dystrophin-positive fibers increased (+) long term residual efficacy over 6 weeks (-) low general transfection efficiency |
I.M. | [163] |
| PEI-PEG/PLGA | DMD | 2′-OMe ASO | Dystrophin pre-mRNA | mdx | (-) no improvement compared to PEI-PEG-ASO | I.M. | [164] | |
| PEI-Pluronic® | DMD | PMO ASO | Dystrophin pre-mRNA | mdx | (+) dystrophin-positive fibers increased up to 4-fold after I.M. (+) dystrophin-positive fibers increased up to 3-fold in all skeletal muscles after I.V. (+) dystrophin-positive fibers increased up to 5-fold in heart after I.V. (+) low muscle tissue, liver and kidney toxicity (-) mild general transfection efficiency |
I.M./I.V. | [165] | |
| DMD | 2′-OMe ASO | Dystrophin pre-mRNA | mdx | (+) dystrophin-positive fibers increased up to 10-fold | I.M. | [166] | ||
| PEG-polycaprolactone PEG-(polylactic acid) |
DMD | PMO ASO | Dystrophin pre-mRNA | mdx | (+) dystrophin-positive fibers increased up to 3-fold (+) low muscle tissue toxicity (-) mild general transfection efficiency |
I.M. | [167] | |
| PMMA | DMD | 2′-OMe ASO | Dystrophin pre-mRNA | mdx | (+) dystrophin-positive fibers increased up to 7-fold (-) slow biodegradability |
I.P. | [168] | |
| PMMA/NIPAM | DMD | 2′-OMe ASO | Dystrophin pre-mRNA | mdx | (+) dystrophin-positive fibers increased up to 4-fold (+) body-wide dystrophin restoration after I.V. (+) exon-skipping level enhanced up to 20-fold (+) long term residual efficacy over 90 days |
I.P./I.V. | [169,170] | |
| PEA | DMD | 2′-OMe ASO | Dystrophin pre-mRNA | mdx | (+) dystrophin-positive fibers increased up to 3–10-fold | I.M. | [171] | |
| DMD | PMO ASO | Dystrophin pre-mRNA | mdx | (+) dystrophin-positive fibers increased up to 3-fold after I.M. (+) body-wide dystrophin-positive fibers increased up to 3-fold after I.V. |
I.M./I.V. | [171] | ||
| Muscle atrophy/ DMD |
pDNA | Cell nucleus | mdx | (+) transfection efficiency enhanced up to 6-fold | I.M. | [172] | ||
| PLys-PEG | Muscle atrophy | pDNA | Cell nucleus | Balb/c | (+) transfection efficiency enhanced up to 10-fold | I.V. | [173] | |
| PPE-EA | Muscle atrophy | pDNA | Cell nucleus | Balb/c | (+) transfection efficiency enhanced up to 13-fold (+) long term residual efficacy over 14 days |
I.M. | [174] | |
| Atelocollagen | Muscle atrophy/ DMD |
siRNA | Cytoplasm | mdx | (+) higher mass muscle increase | I.M./I.V. | [175] | |
| PAMAM-OH | Muscle atrophy | Angiotensin (1–7) | Cytoplasm | Balb/c | (+) higher anti-atrophic effects | I.P. | [176] | |
| Lipidic | PEG-bubble liposomes |
DMD | PMO ASO | Dystrophin pre-mRNA | mdx | (+) dystrophin-positive fibers increased up to 1.5-fold (+) exon-skipping level enhanced up to 5-fold |
I.M. | [177] |
| DM1 | PMO ASO | Clcn1 pre-mRNA | HSALR | (+) increased expression of Clcn1 protein up to 1.4-fold | I.M. | [178] | ||
| Nanolipodendrosomes | DMD | MyoD and GA | Cytoplasm | SW-1 | (+) slight mass muscle increase | I.M. | [179] | |
| Nanoliposomes | DMD | Glucocorticoide | Cell nucleus | mdx | (+) lower inflammatory induced response (+) lower bone catabolic effects |
I.V. | [180] | |
| Hybrid liposomes DMPC and (C12(EO)23) | DMD | Gentamicin | Ribosomes | mdx | (+) dystrophin-positive fibers increased up to 4-fold (+) lower ototoxicity and nephrotoxicity |
I.P. | [181] | |
| Perfluorocarbon | DMD | Rapamycin | mTORC1 complex | mdx | (+) high muscle strength increase (+) high cardiac contractile performance increase |
I.V. | [182] | |
| Lipid NPs | DMD | CRISPR/Cas9 | Dystrophin DNA sequence |
ΔEx44 | (+) dystrophin expression restored up to 5% | I.M. | [183] | |
| Inorganic | Gold | DMD | CRISPR/Cas9 | Dystrophin DNA sequence |
mdx | (+) HDR in the dystrophin gene enhanced up to 18-fold | I.M. | [184] |
3. Future Perspectives
The recent understanding of the pathogenic mechanisms of MDs highlights the urgent need of new and more effective treatments [20,100]. Nanomedicine demonstrated to enhance the therapeutic potential of gene therapy and drug repurposing approaches. As an example, pentamidine-loaded nanomedicines were used to explore the activity of the drug, not only as an anti-leishmaniasis agent but also as an anticancer agent to reduce drug associated toxicity, such as its severe nephrotoxicity [271,272]. Ongoing investigations are aimed at demonstrating the efficacy of this novel formulation to treat DM1 (study in progress).
New therapeutic approaches needed a continuous administration throughout patient’s life, making the biocompatibility and biodegradability of delivery systems a crucial feature to preserve skeletal muscle from additional alterations. As presented in this review, the main advantages of nanosystems rely on their physico-chemical properties, namely composition, size and surface potential that can be modulated to avoid nanocarrier toxicity, and to load specific actives and deliver them in a target site.
To disclose the potential of nanomedicine application to MDs treatment, the gap between in vitro and in vivo testing has to be filled. In addition, to understand the fate of nanosystems once administered to MDs mice, biodistribution studies need to be addressed. To date, only a few investigations reported NPs biodistribution into skeletal muscles through different administration routes as I.V., I.P. and I.M. (tibialis anterior and gastrocnemius muscles) [173,273,274]. After systemic administration, NPs spread into tissues through blood systemic circulation, then, extravasate into the ECM before reaching muscle fibres. It has been suggested that the dense blood capillary network wrapping skeletal myofibers could be favourable to NPs accumulation and distribution following I.V. injection [196]. Hydrodynamic injection is also known to facilitate gene delivery by transient enhancement of the plasma membrane’s permeability [142,275,276]. However, the applied pressure due to the hindrance of the blood flow might cause oedema and inflammation, restricting the translation of this technique to clinic [277].
Overcoming the ECM barrier remains another important goal to improve NPs distribution in skeletal muscle fibres. Surface engineered nanosystems have been designed to actively promote the interaction between nanosystems and cells [278]. The high specificity of antibodies for their corresponding antigen provides a selective and potent approach for therapeutic NPs targeting [279]. As an example, the murine monoclonal antibody (3E10), capable of binding the surface of muscle cells, has been reported to improve active targeting [280,281]. However, no scientific studies on antibody-functionalised NPs have been conducted for skeletal muscle targeting so far. More commonly used, short peptides sequences (e.g., ASSLNIA or SKTFNTHPQSTP) have proved promising as NPs functionalization for specific tissue-targeting [282,283,284]. Several examples of peptides targeting muscle cells have been reported [285,286,287] and association to nanosystems may lead to improved selectivity of NPs for skeletal muscle. Polymeric nanosystems have been functionalised with active targeting agents that preferentially bind active molecules or receptors expressed on the surface of muscle cells. Active targeting-dependent uptake has been demonstrated using PLGA nanocarriers functionalised with a muscle-homing peptide M12 [288]. Biodistribution studies revealed a preferential accumulation of targeted NPs in skeletal muscle cells in mdx mice, compared to untargeted nanocarriers, increasing the accumulation of polymeric NPs and enhancing therapeutic efficacy [274].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13020278
This entry is offline, you can click here to edit this entry!
