During the first wave of the COVID-19 pandemic, industries and academic institutes have col-laborated to resolve the worldwide medical supply shortage issues. Innovative designs of 3D-printed items were proposed and developed by the maker community as a temporary solution to address the lack of personal protective equipment. An overview of global ongoing and past ini-tiatives during the COVID-19 pandemic along with their challenges on retrofitting full-face snor-keling masks for healthcare applications such as splash-proof face shields, respirator masks and non-invasive ventilation systems are reported in this contribution.
- personal protective equipment
- non-invasive ventilation systems
- rapid prototyping
- full-face snorkeling masks
- healthcare products
1. Introduction
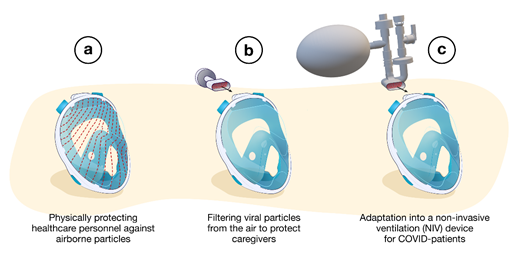
The proposed user strategies for using snorkeling masks in health care are summarized in Figure 1, including their use as a splash-proof face shield, respirator mask and NIV device.
Figure 1. Different strategies used to redesign snorkeling masks for medical use during the COVID-19 pandemic: (a) splash-proof face shields, (b) respirator masks, (c) non-invasive ventilation devices.
2. Splash-Proof Face Shields
In the first case (Figure 1a), the mask is used as a splash-proof face shield and no additional printed item is necessary. The main function of the mask is to limit the flow of relatively large airborne particles (e.g., large droplets of infected saliva (submillimeter sizes)) [1] directed to the face. Of note, splash-proof face shields are less protective of infectious aerosols (<5 µm) [2], as these can be inhaled either by the space between the shield and face or by the inhalation tubes in the case of using a snorkeling mask as splash-proof face shield. However, the advantage of using snorkeling masks is full-face coverage (nose, mouth and eyes), compared to standard face shields, thus limiting self-contamination by hand-to-eye contact. The firm fixation of the snorkeling masks to the head ensures protection during rapid movements (e.g., falling, running, etc.). However, a few practical aspects could limit their use. Snorkeling masks remain uncomfortable (e.g., relatively high resistance for breathing, condensation within the mask) compared to classic face shields. Proper mask handling [3] (e.g., putting on and taking off the mask must be followed by a cleaning procedure) is another concern to comply with the WHO recommendations [4]. Lawrence et al. [5] evaluated the extent of droplet contamination during mastoid drilling in a preclinical model. The authors reported virus contamination extended for over 2 m from the drill site. While, in this work, the protection, the comfort and the breathability with the modified snorkeling mask were validated as adequate/good, some problems were observed. For example, a standard full-face respirator appeared to be more effective for HCW–patient communication compared to a modified full-face snorkel mask [5]. Therefore, most of the participating surgeons stated that they would not personally use the modified snorkeling mask in future treatments/work. Communication issues between coworkers while wearing snorkeling masks were also reported by Palmeiro et al. [6]. To overcome this problem, solutions were proposed, including C-Voice Masks, manufactured by an Italian company named Siropack and the Industrial Engineering Department of the University of Bologna [7]. This device was designed with an audio amplification system to facilitate the communication between HCWs. Similarly, Krool et al. created a mobile application to transmit the voice from a wireless microphone inside the mask to speakers in the environment of use [8].
3. Respirator Masks
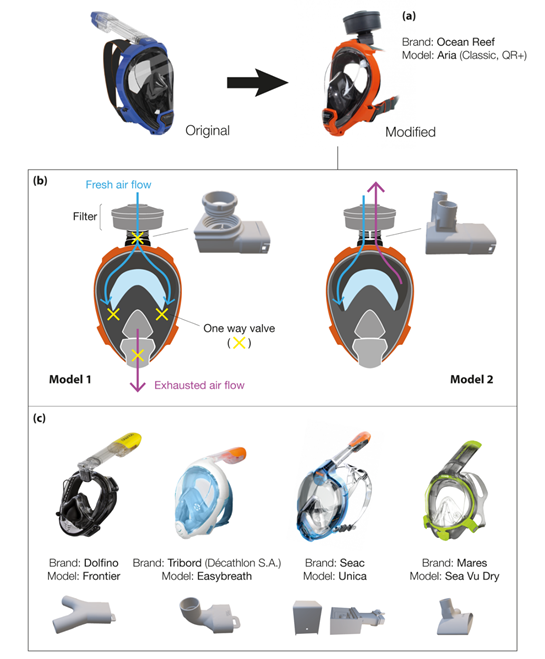
In the second case (Figure 1b), the snorkeling mask is used as a full-face particulate-filtering face-piece respirator. The 3D-printed adapters replace the snorkel exhale channel usually located at the top of the mask. These respirators are known as N95 masks when they conform to Occupational Safety and Health (OSH) standards. Quality N95 filter material is scarce as it should be composed of non-woven melt blown fabric, which is not readily available in many countries. As a result, makeshift masks are equipped with all sorts of alternative filters (i.e., cheap surgical masks, Halyard H600 drapes, generic filters with some high-efficiency particulate air (HEPA) classification). Figure 2a highlights the modifications of the Ocean Reef snorkeling mask, an open source project developed by the Italian company Mestel Safety (Ocean Reef Group) [9]. The company offered the possibility to buy the patented connectors at low prices to help owners convert their snorkeling masks. If needed, 3D printing design files were shared online for non-commercial use [10]. Two coupling adapter prototypes were proposed, which were suitable for different conditions of application (Figure 2b).
Figure 2. (a) Modifications of Ocean Reef’s masks using 3D-printed components; (b) different respirator modes obtained with the Ocean Reef’s masks; (c) most common masks studied by the community during the COVID-19 pandemic.
The first adapter was for general use, where a sterile environment was not required. In this case, the one-way silicone valve (i.e., mushroom valves), present near the mouth/chin area, allows exhausted air to be expelled from the lower part of the mask. This design corresponds more to a respirator mask with a breather valve, which protects the user but does not filter its exhalations [11]. The second adapter filters both the inhaled and the exhaled air and is specifically designed for HCWs. The 3D-printed connector has two separated circuits: one for the intake and one for the exhaust. The one-way silicone valve near the mouth/chin area is blocked (usually with tape or glued shut). An additional breathing valve is placed in the exhaust connector to limit the return air from filters and the dead air space in the mask. Nicholson et al. studied the influence of three experimental configurations of the snorkel mask (i.e., removing the mushroom valve, blocking the valve exhaust, normal conditions) during a medical standard fit test [12]. All analyses were performed following the United States Occupational Safety and Health Administration (OSHA) 29CFR1910 standard [13]. Although the qualitative analysis exceeded the OSHA requirements for all conditions, significant differences were observed between blocking and opening the valve exhaust. This suggested a reduced protection by modified masks when the mushroom valve is in place. In addition, Krool et al. demonstrated that the custom adapter and the chin valve did not generate significant leaks [8]. The authors showed that fit factors corresponded to the requirement for elastomeric half-masks. Another study also confirmed that the modified mask passed the OSHA N95 respirator fit test requirements (fit factors should exceed 100) as a fit factor of 142 was obtained and neither hypercapnia nor hypoxemia occurred. [83] Additional information can also be found from the work of Tack et al. [14]. The authors tested the modified snorkeling mask on 40 healthcare workers (COVID-19-free) during 2 h of normal work. The retrieved data did not show significant changes for blood gas analysis after 120 min. Masks were well within the required tolerances although after 2 h, 17% of the participants reported some difficulties (ratings of perceived exertion (RPE) scored at 5/10). It is important to note that over the last months, several adaptors were developed due to the wide variety of full-face snorkeling mask brands [8]. This has increased the number of immediate transformable protection equipment to two million pieces (considering the available stock to date) [9]. The quality and the price of these full-face masks vary depending on the geographical area and the brand. For example, in the USA, prices range from 20 to 90 USD for a single mask, with a mean cost of approximately 56 USD [8]. Figure 2c outlines the four most common brands studied by the community during the COVID-19 pandemic, together with their developed connectors to use these snorkeling masks as respirator masks. Vicini et al. tested five different modified masks during otolaryngological surgery and in anesthesia procedures on COVID-19-free healthcare professionals [15]. The deployed modified devices were adapted from the following brands:
- Subea Easybreath (Decathlon, France);
- Seac Unica (Seac Sub, Italy);
- Siropack C-Voice, (Siropack, Italy);
- Ocean Reef Aria QR+ (Mestel Safety, Italy).
The authors concluded that a more customized mask is necessary to fulfill the medical and surgical needs. As an example, the authors suggest modifying the system to allow the use of personal glasses inside the mask, including a microphone/loudspeaker system and integrating a lamp as well as a magnifying loupe system, all necessary for well-functioning of most medical procedures. Similarly, Dalla et al. reported a study on modified Ocean Reef snorkel masks [16]. Over 22 masks were fit-tested on healthy individuals. The authors report that 86.9% (n = 459) of the models that passed qualitative fit tests (QLFT) also passed quantitative fit testing. Among the wide variety of proposed designs for snorkeling mask retrofitting for use as PPE, pioneering work was conducted by the Prakash team at Stanford, together with Dolfino Frontier® designed mask connectors integrating standard commercial filters (medical or industrial grade) [17]. To use industrial National Institute for Occupational Safety and Health (NIOSH)-approved filters, an additional modular component was designed, and a proper gasket was mounted in the assembly. The results obtained from fit tests changed depending on the analyzed area in the mask. [17]. The same results were obtained when the sampling tube was placed close to the mouth or in the eyes chamber. In contrast, significant higher leaks were found when the tube was connected to the 3D-printed adapter. The authors concluded that leaks depend on how well the mask fits the wearer’s face. Thus, results vary depending on the combination of face and mask shapes. Regarding materials, Prakash et al. report working mostly with a biocompatible polyethylene resin (Carbon RPU-70 from PrinterPrezz [18]) [17]. The Grey Pro Resin (from Formlabs) was also used due to its good mechanical performance [19]. However, the former had issues regarding achieved tolerances, so a careful check during the assembling steps was suggested. Moreover, fused deposition modeling (FDM) may result in porous connectors, which remain dangerous in the presence of viral gases (video [20]). Previous studies identified key parameters affecting the final quality of different 3D-printed objects [21], such as the wall permeability depending on the geometric shape, the filament feed rate and the predefined (G-code) wall structure. Although most of the investigations suggested that performance and efficiency of the tested masks were comparable or exceeded the N95 standard, all authors recommended caution as the adaptors were not certified for medical use and companies as well as inventors did not guarantee the safety of the protection device (mask + connector). Last April, the FDA issued an updated guidance addressing these issues [22]: when alternatives, such as FDA-cleared masks or respirators, are unavailable, individuals (including healthcare professionals) might improvise PPE. Similarly, the National Medicines Safety Agency, the French version of the FDA (ANSM), authorized the distribution of adapters in France until 31 May 2020, as the material ensuring the same protection guarantees and meeting the compliance requirements was not available [23]. Another preliminary study conducted in Canada outlined the challenges for retrofitting N95 filters on snorkeling masks [24]. Based on the Centers for Disease Control and Prevention (CDC) Guidelines on Crisis/Alternate Strategies for PPE decisions in the setting of N95 respirator shortages [25], the modified snorkeling masks can be considered as “healthcare personal use of non-NIOSH-approved masks or homemade masks”.
4. Non-Invasive Ventilation Devices
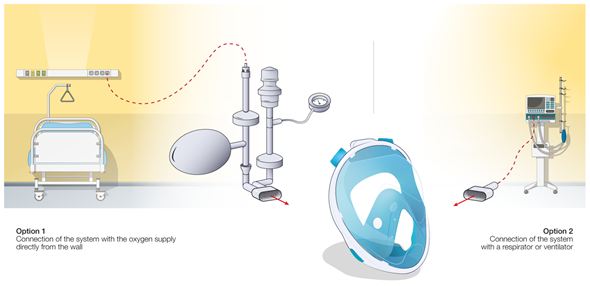
In the third case (Figure 1c), standard snorkeling masks have been modified into non-invasive ventilation (NIV) devices for the treatment of acute hypoxemic respiratory failure of COVID-19 patients [26]. These modified masks (MM), by adequately replacing the unexpected shortage of the standard medical ventilation devices and masks, ensured a positive airway pressure during the entire respiration cycle and reduced the intubation rates [27]. The MM requires disposable tubing components along with 3D-printed connectors. Different configurations have been proposed as presented in Figure 3.
Figure 3. Different configurations of snorkeling mask usage and developed connectors as non-invasive ventilation devices.
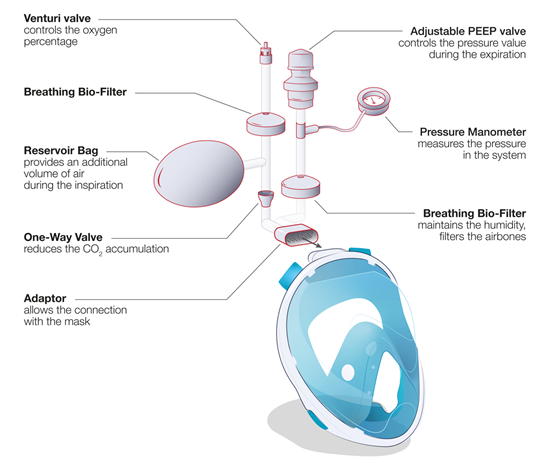
In a conventional ventilation method, the standard medical mask is connected directly to a CPAP module (e.g., WhisperFlow 2–60 from Respironics). This situation of direct connection of a mask to a respirator/ventilator device is outlined in Figure 3, option 2, for the case of retrofitted snorkeling modified masks (MM) using the developed (mostly 3D-printed) connectors. Alternatively, the system can be connected directly to the pressure-regulated oxygen source of the hospital (deployed pressures are usually between 3.5 and 50 bars before the pressure regulator) and an oxygen air mixer can be placed between the mask tubing and the source of oxygen (e.g., EasyMIX® from Flow-Meter S.p.A). Some authors also reported the possibility to connect the system directly to the oxygen source without any flowmeter [28], having the oxygen supply controlled by a Venturi valve (e.g., 0060000 from Intersurgical) typically used during oxygenation therapy. Hence, the tubing and the design of the NIV MM were adapted to ensure correct operation of the system (Figure 3, option 1). The majority of hospitalized COVID-19 patients require high fraction of inspired oxygen (FiO2) during NIV [29]. However, the highest FiO2 that can be obtained via commercial Venturi valves is usually limited to 60%. In order to increase the FiO2, a modified device using a Venturi valve with a secondary inlet was suggested [99], as shown in Figure 4.
Figure 4. Full-face snorkeling mask adapted for use as a non-invasive ventilation device: schematic of the different components when using a Venturi valve and function of each element.
Most of the MM designs used commercial medical tubes (e.g., 1528000, 22 mm Flextube™, 1.6 m long, Intersurgical) to connect the mask to an available gas supply. An adjustable positive end-expiratory pressure (PEEP) valve (e.g., item n. 2226000, 2.5–20 cm H2O, 22 m, Intersurgical) was also integrated to control the positive pressure inside the mask. To limit the spread of the virus outside the closed circuit, two breathing filters (e.g., item n. 1544007 Clear Guard™3, Intersurgical) were positioned at the entrance and exit of the circuit. Some of the MMs used a reservoir bag placed in the inlet (e.g., 2820000 Reservoir bag, 2 L with antiocclusion cage mount, 22 F neck, Intersurgical) to increase the air volume and pressure during the first seconds of inspiration. A one-way directional valve (e.g., item n. 1921000 22M flow in, 22F flow out, Intersurgical) was often placed between the mask and the entrance of the gas flow to reduce the dead space. This one-way directional valve is placed after the bag reservoir to decrease any CO2 accumulation during the respiratory cycle. A disposable manometer (e.g., item n. 7162000, Intersurgical) could also be connected to the circuit to measure the internal pressure for improved system control. Different commercial adaptors were adopted (i.e., 1568000 straight connector 22F, 6 mm oxygen stem, Intersurgical) to fit the different items together. Actual case studies conducted on virus-infected patients are scarce in literature. Wagner et al. [30] reported the use of a modified snorkeling mask as a NIV device during spontaneous breathing on a female patient, 56 years old, with SARS-CoV-2 infection resulting in the coronavirus disease (COVID-19), and having systemic arterial hypertension and obesity. The mask was used two times/day for 40–60 min, combined to a mechanical ventilator until day 12 of hospitalization. This work demonstrated that the snorkeling mask during spontaneous breathing is effective to prevent orotracheal intubation (i.e., the use of invasive ventilation system). However, a comparative study with a commercial medical mask and a larger number of cases are necessary to support and statistically validate this study. Another study was performed by Bibiano-Guillen et al. at a Spanish medical center, Hospital Universitario Infanta Leonor of Madrid [31]. They deployed ventilation therapy using modified snorkeling masks, which were tested interruptedly on 25 patients having acute respiratory syndrome secondary to SARS-CoV-2 infection. In all studies, no hypercapnia or respiratory acidosis was observed in any patient, meaning that rebreathing did not take place. For this reason, the authors conclude that the use of modified snorkeling masks “complies with clinical requirements to be produced in other centers with limited resources, especially in developing countries” if there is a “deep shortage of certified alternatives” but the users must be “aware of the limitations”. To date, additional tests, made with a more statistical and medical methodology, seem necessary to highlight the relevance and the safety of this approach.
This entry is adapted from the peer-reviewed paper 10.3390/healthcare9020204
References
- Pendar, M.R.; Páscoa, J.C. Numerical Modeling of the Distribution of Virus Carrying Saliva Droplets during Sneeze and Cough. Phys. Fluids 2020,
- Fennelly, K.P. Particle sizes of infectious aerosols: Implications for infection control. Lancet Respir. Med. 2020, 8, 914–924.
- Thierry, B.; Célérier, C.; Simon, F.; Lacroix, C.; Khonsari, R. How and why use the EasyBreath® surface snorkeling mask as a personal protective equipment during the COVID-19 pandemic? Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020, 137, 329–331.
- World Health Organization(WHO). How to Put on, Use, Take off and Dispose of a Mask. Available online: https://www.who.int/docs/default-source/epi-win/how-to-use-mask-v0-1-print.pdf?sfvrsn=64ba1493_2 (accessed on 20 September 2020).
- Lawrence, R.J.; O’Donoghue, G.; Kitterick, P.; O’Donoghue, K.; Hague, R.; Mitchell, L.; Lycett-Ranson, Z.; Hartley, D.E.H. Recommended Personal Protective Equipment for Cochlear Implant and Other Mastoid Surgery During the COVID-19 Era. Laryngoscope 2020, 130, 2693–2699.
- Palmeiro, A.J.; Symons, D.; Morgan III, J.W.; Shaffer, R.E. Speech intelligibility assessment of protective facemasks and air-purifying respirators. J. Occup. Environ. Hyg. 2016, 13, 960–968.
- SiroPACK C-Voice Mask Contro COVID-19. Available online: http://www.siropack.it/c-voice-mask-contro-covid-19/#ita
- Kroo, L.; Kothari, A.; Hannebelle, M.; Herring, G.; Pollina, T.; Chang Md, R.; Banavar, S.P.; Flaum, E.; Soto-Montoya, H.; Li, H.; et al. Pneumask: Modified Full-Face Snorkel Masks as Reusable Personal Protective Equipment for Hospital Personnel. medRxiv 2020.
- OceanReef Group. Certified Adaptations of Full Face Masks to Address COVID-19 CRISIS. Available online: https://oceanreefgroup.com/COVID19 (accessed on 20 September 2020).
- OceanReef Group 3D Printing Files. Available online: https://oceanreefgroup.com/covid-19-3d-printing/#privatedownload-form-expanded-use (accessed on 20 September 2020).
- Center for Disease Control and Prevention Personal Protective Equipment: Questions and Answers. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirator-use-faq.html (accessed on 28 September 2020).
- Nicholson, K.; Henke-Adams, A.; Henke, D.M.; Kravitz, A.V.; Gay, H.A. Modified Full-Face Snorkel Mask as COVID-19 Personal Protective Equipment: Quantitative Results. Preprints 2020. Available online: https://www.preprints.org/manuscript/202004.0293/v1 (accessed on 13 February 2021).
- United States Departement of Labor Regulations (Standards—29 CFR). Available online: https://www.osha.gov/laws-regs/regulations/standardnumber/1910 (accessed on 20 September 2020).
- Tack, P.; Mylle, M.; De Vleesschouwer, M.; Fevery, S.; De Gersem, R.; Lemmens, P.; Cruyt, N.; Devloo, R.; Van Kerkhove, O.; Lanckmans, L.; et al. Testing of Safety and Ease of Use of an Adjusted Full Face Snorkel Mask as Protective Measure for Caregivers During the COVID-19 Pandemic—MASK Study. Lancet 2020.
- Vicini, C.; Cammaroto, G.; Meccariello, G.; Iannella, G.; Fragale, M.; Cacco, T.; Sampieri, C.; Guastini, L.; Castello, E.; Parrinello, G.; et al. Overview of different modified full-face snorkelling masks for intraoperative protection. Acta Otorhinolaryngol. Ital. 2020, 40, 317–324.
- Dalla, S.; Shinde, R.; Ayres, J.; Waller, S.; Nachtigal, J. 3D Printed Snorkel Mask Adapter for Failed N95 Fit Tests and PPE Shortages Shiv. medRxiv 2020.
- Kroo, L.; Kothari, A.; Hannebelle, M.; Herring, G.; Pollina, T.; Chang Md, R.; Banavar, S.P.; Flaum, E.; Soto-Montoya, H.; Li, H.; et al. Pneumask: Modified Full-Face Snorkel Masks as Reusable Personal Protective Equipment for Hospital Personnel. medRxiv 2020.
- Carbon3d 3D Printing Materials for Real-World Applications. Available online: https://www.carbon3d.com/materials/rpu-rigid-polyurethane/ (accessed on 20 September 2020).
- Formlabs Material Data Sheet for Grey Pro. Available online: https://formlabs-media.formlabs.com/datasheets/Grey_Pro_Technical.pdf (accessed on 20 September 2020).
- PPE-Pneumask Danger of Monofilament 3D Printing—Pneumask. Available online: https://www.youtube.com/watch?v=1m8VTdZwjVE (accessed on 20 June 2020).
- Gordeev, E.G.; Galushko, A.S.; Ananikov, V.P. Improvement of quality of 3D printed objects by elimination of microscopic structural defects in fused deposition modeling. PLoS ONE 2018, 13.
- U.S. Department of Health and Human Services Food and Drug (CDRH) Center for Devices and Radiological Health Office of Product Evaluation and Quality (OPEQ). Enforcement Policy for Telethermographic Systems During the Coronavirus Disease 2019 (Covid-19) Public Health Emergency Guidance for Industry and Food and Drug Administration Staff. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-telethermographic-systems-during-coronavirus-disease-2019-covid-19-public-health (accessed on 12 June 2020).
- Planktonplanet Masque-Adaptateur COVID-19 Consortium France. Available online: https://adaptateur-masque.planktonplanet.org/ (accessed on 20 September 2020).
- Bailly, N.; Petit, Y.; Wagnac, E.; Hof, L.A.; Lau, C.; Vandenbroucke, J.-É.; Brailovski, V.; de Guise, J.; Desrosiers, J.-M.; Laroche, É.; et al. Projet De Masques De Protection Individuelle Reutilisables A Partir De Masques De Plongee; ÉTS: Montréal, QC, Canada, 2020.
- Center for Disease Control and Prevention Optimizing N95 Respirator Supplies. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirators-strategy/index.html (accessed on 27 September 2020).
- Groenhuis, V. Silvana Decathlon Face Mask Adapters. Available online: https://www.prusaprinters.org/prints/27214-silvana-decathlon-face-mask-adapters (accessed on 20 September 2020).
- Alharthy, A.; Faqihi, F.; Noor, A.F.; Soliman, I.; Brindley, P.G.; Karakitsos, D.; Memish, Z.A. Helmet Continuous Positive Airway Pressure in the Treatment of COVID-19 Patients with Acute Respiratory Failure could be an Effective Strategy: A Feasibility Study. J. Epidemiol. Glob. Health 2020, 10, 201–203.
- Isinnova Web Site. Available online: https://www.isinnova.it (accessed on 10 March 2020).
- BC Critical Care COVID-19 Comitee. CRG 27_High-Flow Oxygen During the COVID-19 Pandemic; BC Critical Care COVID-19 Comitee: British Columbia, BC, Canada, 2020.
- Wagner, L.E.; Basegio, K.G.; Dornelles, C.F.D.; Foernges, R.; Gaedke, M.Â.; da Silva, A.L.G.; Paiva, D.N. Diving Mask Adapted for Non-Invasive Ventilation and Prone Position in a Patient with Severe Covid-19: Case Report. J. Epidemiol. Infect. Control 2020, 39.
- Bibiano-Guillen, C.; Arias-Arcos, B.; Collado-Escudero, C.; Mir-Montero, M.; Corella-Montoya, F.; Torres-Macho, J.; Buendía-Garcia, M.J.; Larrainzar-Garijo, R. Adapted Diving Mask (ADM) device as respiratory support with oxygen output during COVID-19 pandemic. Am. J. Emerg. Med. 2021, 39, 42–47.