Cognitive decline refers to a deterioration of intellectual and learning abilities and related memory problems, and is often associated with behavioral alterations, which prevents sufferers from carrying out the most common daily activities, such as maintaining normal productive interpersonal relationships, communicating, and leading an autonomous life. Numerous studies have highlighted the association between cognitive decline and autoimmune disorders, including rheumatoid arthritis (RA). RA is a chronic, inflammatory, autoimmune disease that involves systems and organs other than the bones and joints, with varying severity among patients.
- rheumatoid arthritis
- cognitive decline
- pathogenesis
1. Rheumatoid Arthritis (RA)
RA is a chronic, inflammatory, autoimmune disease that involves systems and organs other than the bones and joints, with varying severity between patients [1]. RA is characterized by symmetrical joint pain associated with morning stiffness (joints are affected for >30 min), hyperplasia (swelling), and cartilage and bone destruction that causes rheumatoid nodules under the skin (deformity) [2,3]. However, RA not only affects the joints but is also associated with secondary amyloidosis, lymphomas, cardiovascular and pulmonary disease, vasculitis, and psychological and skeletal disorders that may cause permanent disability in many instances [2]. The presence of RA is relatively constant in the global population, with a prevalence between 0.5% and 1.0% in the European and North American populations [4]. Twice as many women are affected than men, and although it is more common in people in their fifties, it can appear at any age [4].
A complex interaction between genotype and exposure to environmental factors (cigarette smoking, air pollutants, and occupational dust) is likely to determine the onset and development of RA [5]. Genetic studies have found an association of more than 100 polymorphisms with RA, mainly in the major histocompatibility complex (MHC) locus, but also in genes encoding for cytokines/chemokines and their receptors, components of intracellular signaling pathways, and costimulatory factors [6]. The implication of genetic factors in the RA pathogenesis is demonstrated by a positive family history, which increases the risk of RA by about three to five times [4,7]. Along the same lines, a concordance rate of 15% to 30% is observed among monozygotic twins, while only a 5% concordance can be observed among dizygotic twins [1]. The characteristic chronic inflammation of the joints suggests an autoimmune origin of the pathology [8]. Typical histological findings are the symmetrical synovial proliferation, with the destruction of cartilage and bone damage induced by the activation of self-reactive T and B lymphocytes, which produce proinflammatory cytokines and autoantibodies [9] (Figure 1). Evidence obtained from preclinical and clinical studies from animal models of RA and RA patients demonstrates that CD4+ T cells belonging to the proinflammatory subgroups Th1 and Th17, together with M1 macrophages, contribute to the development and maintenance of RA and counteract the action of Th2 and Th3 anti-inflammatory cells and M2 macrophages [8].
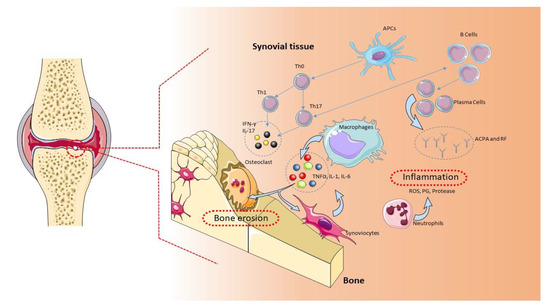
Figure 1. Pathogenic role of immune cells in rheumatoid arthritis (RA). The immune cells mainly involved in the pathogenesis of RA are B cells, T cells, and macrophages. These cells are normally present in the synovial tissue. B cells release proteins, such as rheumatoid factor (RF), protein antibodies (ACPAs), and proinflammatory cytokines, that support the establishment of RA. B cells also mediate the activation of T lymphocytes through the expression of costimulatory molecules. In RA, the main function of T lymphocytes is to activate macrophages. Activated T lymphocytes and macrophages release proinflammatory molecules, such as cytokines and chemokines, which keep the osteoarticular tissue inflamed. This condition favors the activation of synoviocytes and osteoclasts, with consequent damage to the osteoarticular tissue and pannus formation [1]. APCs: antigen-presenting cells, ROS: reactive oxygen species.
2. Diagnosis and Treatment of RA
RA is a symmetrical polyarthritis with a gradual and persistent chronic course that primarily involves the joints of the hands and feet [4]. The key features of inflammatory arthritis are the presence of early morning stiffness, joint swelling affecting more than three joints, and tenderness across the metacarpo- and metatarso-phalangeal joints, as evaluated by the “squeeze test.” Axial joint involvement is less common, with cervical spine involvement occurring in 30–50% of cases, but rarely in isolation. Temporo-mandibular and crico-arytenoid joints may also be affected [10]. However, the clinical presentation and the course of the disease vary among patients, with some having very acute onset polyarthritis [4].
The European League Against Rheumatism (EULAR) Disease Activity Score (DAS-28) includes the evaluation of the number of affected joints, the level of acute inflammatory markers, and the patient global well-being score [10]. RA is characterized by the presence of rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPAs). Positive serology for RF can be found in 60–80% of patients with established RA, but it is less frequently present in early RA (<50%). ACPAs have a specificity of 94–97% and a sensitivity of 62–72% in early RA. ACPAs can be detected early in the course of RA, often appearing before RF, and may be observed before clinical manifestations of the disease. In ACPAs-negative patients, other autoantibodies have been observed, including anti-carbamylated proteins [11], anti-malondialdehyde acetaldehyde [12], anti BRAF [13], 14-3-3eta, anti-CarP, anti-Sa [14], and anti PAD3/PAD4 [15] antibodies.
Non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen, naproxen, ketoprofen, piroxicam, diclofenac, and Celecoxib, are widely used as symptomatic therapies [16]. Disease-modifying antirheumatic drugs (DMARDs) are a heterogeneous collection of agents that represent the mainstay of treatment for RA [16]. DMARDs can be classified as conventional synthetic DMARDs (csDMARDs, which include methotrexate, sulfasalazine, leflunomide, and hydroxychloroquine), targeted synthetic DMARDs (tsDMARDs, which include tofacitinib and baricitinib), and biological DMARDs (bDMARDs, such as adalimumab, tocilizumab, secukinumab, abatacept, and anakinra). Methotrexate represents the first choice csDMARD. Leflunomide or sulfasalazine is used in the case of contraindication to methotrexate. In patients not responding to treatment, or in the presence of poor prognostic factors, it is recommended to add a bDMARD or tsDMARD. Steroids, although effective in reducing pain and disease progression, are used temporarily as an adjunctive treatment because of their side effects [17].
3. Cognitive Decline
Cognitive decline can be defined as a psychophysical condition that is characterized by an alteration in the orientation, attention, problem-solving abilities, memory, and executive functions [18]. Cognitive impairment may range from a subtle decline in a single cognitive domain to impairment in multiple cognitive domains (mild cognitive impairment (MCI)) to frank dementia, which is characterized by cognitive decline and the loss of function. To diagnose MCI, the following parameters should be met: complaint of a decline in cognitive function, impairment of one or more cognitive domains, and independent function preserved, with no alteration in social and work skills [19].
Impaired episodic memory is typically seen in patients with MCI, who may later progress to dementia. Alzheimer’s disease (AD) is the most common form of dementia and accounts for 50–70% of dementia cases [20].
The growth of the aging population has been associated with an increased burden from cognitive disorders. The prevalence of MCI ranges from 12% to 18% among the older adults (≥65 years old) with an annual 10–15% conversion rate to AD [20].
Cognitive impairment can arise from many chronic diseases, such as hypertension, dyslipidemia, vascular disease, diabetes, chronic obstructive lung disease, depression, anxiety, autoimmune diseases, epilepsy, and drug dependency. Head injuries can lead to impaired cognition. Furthermore, anti-depressants, anticonvulsants, and antipsychotics are associated with cognitive decline. However, in most of these conditions, cognitive disorders are treatable, particularly when they are detected early through monitoring.
For the diagnosis of cognitive decline, different standardized tests have been developed, including the Mini-Mental State Examination (MMSE), the Montreal Cognitive Assessment (MoCA), the Trail-Making Test (TMT), the Victoria Stroop Test (VST), the Wechsler Adult Intelligence Scale (WAIS), and the Benton Visual Retention Test (BVRT) [21]. The Beck Depression Inventory (BDI) and the State-Trait Anxiety Inventory (STAIT/S) are used to assess the presence of depression and anxiety, which are commonly found in RA patients [21].
4. RA and Cognitive Decline
An increased risk of cognitive decline has recently been associated with the presence of rheumatic diseases [22,23]. It is hypothesized that the triggering cause could be represented by the systemic inflammation that is associated with a chronic rheumatological condition [22,23]. In particular, numerous studies have shown the presence of a cognitive decline in RA patients [18].
To date, the molecular pathogenetic mechanisms that underlie the association of cognitive decline and RA are not fully clarified. However, during the last few years, a growing number of studies have investigated the link between these conditions, highlighting the potential pathogenic role of several clinical, psychological, and biological factors (Figure 2). These include cardiovascular complications and chronic pain, along with the involvement of autoimmune and inflammatory factors, changes in hormone levels, drug side effects, genetic factors, and psychiatric disorders [18,22,24,25,26].
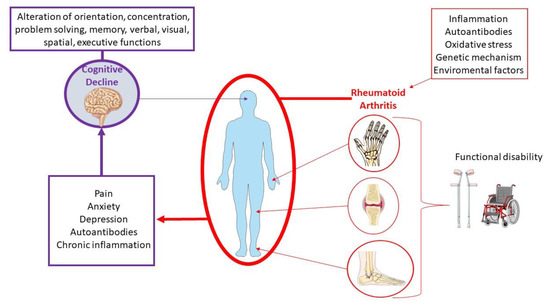
Among the psychiatric conditions, depression and anxiety are mostly associated with RA patients [27]. Usually, the peak of onset of the disease occurs in individuals during their professional and social life, thus compromising the social sphere [28,29]. It has been observed that depression affects up to 66% of patients, anxiety affects 70% of patients, and nearly 17% of RA patients have a major depressive disorder [30,31,32,33]. Depression is usually associated with higher levels of pain and disability, resulting in a lower health-related quality of life and increased mortality [34,35]. Moreover, RA disease activity has been associated with cognitive decline [36,37].
Several studies have reported that the association between cognitive decline and RA is more evident in patients of advanced age [38,39,40,41]. A debilitating condition leading to cognitive decline is associated with the recorded increase in accelerated inflammatory atherosclerosis with a consequent risk of stroke, especially in elderly RA patients with long-standing illness [42]. However, cognitive decline has also been observed in young RA patients, in particular during the early stages of the disease [43].
Other risk factors for cognitive decline in RA include cardiovascular risk and the use of certain drugs, such as glucocorticoids [38]. However, there are some studies that found no association between cardiovascular risk or medication intake and cognitive decline in RA patients [36,44]. On the other hand, treatment with biological anti-tumor necrosis factor (TNF) therapies in RA patients may be protective, showing a lower risk percentage of developing cognitive decline [39].
In this review, we analyzed and explored the correlation between cognitive decline and RA from a pathogenic point of view, focusing on the main molecular mechanisms involved.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22031185
