Photobiomodulation (PBM) therapy employs light at red and near-infrared wavelengths to modulate biological activity. The therapeutic effect of PBM for the treatment or management of several diseases and injuries has gained significant popularity among researchers and clinicians, especially for the management of oral complications of cancer therapy. This entry focuses on the current evidence on the use of PBM for the management of a frequent oral complication due to cancer therapy—taste alteration.
- dysgeusia
- cancer complications
- photobiomodulation
- oral mucositis
- laser therapy
- Taste altera-tion
1. Introduction
Taste is one of the five basic senses, which also include hearing, touch, sight, and smell[1]. The three primary functions of this complex chemical process are pleasure, defense, and sustenance[1][2]. It is the perception derived from the stimulation of chemical molecule receptors in some specific locations of the oral cavity to code the taste qualities, in order to perceive the impact of the food on the organism, essentially[1][2]. An alteration of this typical taste functioning can be caused by various factors and is usually referred to as taste impairments, taste alteration, or dysgeusia[3][4].
In cancer patients, however, the impact of taste alteration or dysgeusia on the quality of life (QoL) is substantial, resulting in significant weight loss, malnutrition, depression, compromising adherence to cancer therapy, and, in severe cases, morbidity[5]. Despite the contemporary thinking of taste alteration’s pathophysiology, the exact mechanism of action in cancer patients is still not entirely understood[5]. Depending on both the cancer and the treatment’s types, the reported prevalence of dysgeusia in cancer patients varies between ±56 to 76%[5][6]. Cancer therapy and, particularly, chemotherapy, radiotherapy, and their associations have been associated within different degrees to cause numerous oral complications, such as the inflammation of the oral mucosa (oral mucositis), the impairment of swallowing (dysphagia), taste alteration (dysgeusia), and the inflammation of the skin (dermatitis), trismus, lymphedema, osteonecrosis of the jaw and others[7]. Nevertheless, preventive and curative methods for the management of these oral complications are still limited, and international guidelines and recommendations are still in need[7][8]. In this entry, a promising and relatively new approach for the therapeutic and curative management of taste alteration will be assessed: photobiomodulation therapy.
2. Photobiomodulation Therapy
In 1917, Albert Einstein enounced the theory of light amplification. It was not until 1960 that Theodore H. Maiman at Hughes Research Laboratories, based on theoretical work by Charles Hard Townes and Arthur Leonard Schawlow, published the first light amplification by stimulated emission of radiation (LASER). In 1967, Andre Mester discovered that a low-power laser had a stimulating effect on hair growth in an experiment aiming to cure malignant tumors implanted in mice. Since then, a plethora of studies strived to achieve and mimic the stimulation effect of the laser obtained by Mester[9][10].
The North American Association for Photobiomodulation Therapy (NAALT) and the World Association of Photobiomodulation Therapy (WALT) define the term photobiomodulation to describe all forms of light that modulate biological activities with a non-ionizing, non-thermal effect[11]. The light sources include laser diodes, light-emitting diodes, and broadband light. In addition, in 2015, the word photobiomodulation was introduced as a Medical Subject Headings (MESH) word in PubMed[11].
In supportive care of cancer, photobiomodulation (PBM) has gained popularity after a plethora of preclinical and clinical studies[7][10]. This plethora of studies has led the Multinational Association of Supportive Cancer Care (MASCC) and the International Association for Oral Oncology (ISOO) to recommend the use of PBM for the management of the oral mucositis (inflammation of the oral mucosa) under chemotherapy and/or radiation therapy, and for hematopoietic stem cell transplantation patients[12]. In addition, in 2018, the National Institute for Health and Care Excellence (NICE) in the United Kingdom recommended the use of PBM therapy for the prevention and treatment of mucositis caused by radiotherapy or chemotherapy[11]. The use of photobiomodulation therapy for the management of taste alteration and other oral complications in cancer patients needs more explorations. Researchers can consider this in future investigations.
2.1. PBM Mechanism of Action
It is now well established that PBM acts principally on the chromophore cytochrome C oxidase (Cox), the terminal enzyme of the electron transport chain, mediating the electron transfer from cytochrome C to molecular oxygen. Distinct evidence shows that if certain conditions are fulfilled, Cox can act as a photo-acceptor and transducer of photo-signals in the red and near-infrared regions of the light spectrum, which can result in a modulation of biological activity. PBM was therefore suggested to increase the availability of electrons for the reduction of molecular oxygen in the catalytic center of Cox, increasing the mitochondrial membrane potential (MMP) and the levels of adenosine triphosphate (ATP), cyclic adenosine monophosphate (cAMP), and reactive oxygen species (ROS)[13].
Furthermore, the action of infrared light on water dynamics in membranes, mitochondria, and/or cells could modulate signaling pathways, the production of reactive oxygen species (ROS), adenosine triphosphate (ATP), Ca2+, NO, and the inositol phosphates group. ROS are known to be effective in brief exposures with low light energy but damaging at high energy levels and chronic, long-term exposure[13][14]. Additionally, it was suggested that the absorption of PBM light causes a short, transient burst of ROS that is followed by an adaptive reduction in oxidative stress. This action is more likely to mitigate radiation-induced injury and mimic the activity of molecular agents that attenuate tissue damage; this leads to the expression of stimulatory and protective genes, which generate growth factors belonging to the fibroblast growth factor family, pro-inflammatory cytokines, and chemokines that are involved in tissue repair[7][15].
2.2. PBM Dosimetry and Parameters
Treatments with PBM will not be reproducible, and outcomes will not be consistent if there is no adequate understanding of the laser–tissue interaction. Various factors, such as the effective energy received by the chromophores and the tissue state, influence the therapeutic effects of PBM[7][16]. A common misconception is that wavelength and energy density (J/cm2) are all that is necessary in order to replicate a successful treatment[17]. In fact, the wavelength, the original power, power density, energy density, and time of exposure must be properly adjusted in order to have a successful treatment. Therefore, even if an adequate device with an adequate wavelength was used, but with doses lower than optimal, PBM will be inefficient. Conversely, if an adequate device with adequate wavelengths was used with higher doses than required, the intervention could be inefficient or even harmful. In fact, a fundamental principle has emerged called the “biphasic dose-response”, where excessive doses of light have been found to be less effective, if not harmful than smaller doses. On the other hand, studies elucidated that the outcome may vary among different cell types and tissue states, for example, healthy versus stressed or hypoxic[17][18]. On the other hand, if the PBM is delivered with higher powers and larger spot sizes, the same energy densities could be delivered to multiple zones at the same time. This can reduce the total time required to cover a large treated area. The same treatment will require less time and become more clinically acceptable.
Hence, adequate dosimetry and comprehension of the laser–tissue interaction are crucial for PBM therapy; this is why, in order to have reproductive studies and better healing, experts in the area of supportive care in cancer and photobiomodulation should work to standardize the parameters for the management of each oral complication of cancer therapy. In this entry, a specific protocol and dosimetry are suggested for taste alteration.
3. Taste Physiology and Pathophysiology in Cancer Patients
It is well acknowledged that humans are chemoheterotrophic organisms[1]. This means that they ingest and digest a wide range of chemical compounds mediated principally by the two chemical senses, taste and smell[1][19]. For millions of years of evolution, the human taste system has developed in a way to identify food’s effects on the organism, which is directly related to humans’ evolution and survival history[20]. Humans possess five basic tastes of sweet, umami, salty, bitter, and sour. Sweet substances are greatly valued and include carbohydrates, the most important source of energy[20]. While umami taste is considered a source of proteins, salty taste is appreciated to maintain the concentration of sodium at balanced levels. Sour taste is, indeed, related to an unripe fruit or spoiled food, due to uncontrolled fermentation. The bitter taste is, nevertheless, accepted in low concentrations due to its positive actions on health[4]. The presence of only five basic tastes has always been a debate between scientists; however, the hypothesis that humans perceive four or five fundamental taste qualities is commonly accepted, and is still practically used in all taste-related studies[4].
In humans, tastes are detected by specific transmembrane receptors that are located at the apex of the taste receptor cells. These taste chemoreceptors are present within the taste buds, which are structures distributed in the different papillae of the tongue and the soft palate. Taste chemoreceptors are specialized epithelial cells located in taste buds, each containing over 150 receptor cells, along with immature cellular elements, the basal cells, and numerous supporting cells[21]. These chemoreceptors are found in the lingual papillae, most prominent on the anterior, lateral, and posterior surfaces of the tongue, in the soft palate, and the oropharyngeal region of the oral cavity[21]. About 1000 taste buds are found on the circumvallate papillae, 12 taste buds are found on the foliate papillae, and only a few taste buds are found on the fungiform papillae. The filiform papillae, the most common, do not contain any taste buds and are involved only in tactile perceptions. The stimulation of the receptors results in a transmission of the message to the regions of the central nervous system, insula, and frontal operculum through the cranial nerves VII, IX, and X[11]. The idea that each papilla represents a receptor for a specific taste has been proven wrong[22][23][24].
Pathophysiology in Cancer Patients
In 1959, McCarthy Leventhal was the first to describe taste alteration under the term “taste hallucinations” in head and neck cancer patients who underwent radiation therapy[25]. The mechanism of action of taste alteration in cancer patients is still not fully understood. However, it is believed that chemotherapy and radiotherapy may cause taste alteration by destroying the rapidly dividing taste bud cells and olfactory receptor cells, and that direct neurologic toxicity may also be involved because the recovery delays epithelial recovery and may continue indefinitely[26]. It is noteworthy to mention that the pathophysiology of dysgeusia differs between chemotherapy and head and neck radiation therapy, and a large number of factors might interplay, resulting in taste alteration[7][26][27][28]. For instance, some studies suggested that hyposalivation might have a significant contribution to taste alteration[28]. On the other hand, the presence of the anterior part of the tongue in the radiation field may also be a predictive factor[29].
Cancer therapy-induced dysgeusia is a frequent complication. Its occurrence was noted in 76% of cancer patients who underwent chemotherapy and radiation therapy, and 66% following head and neck radiotherapy alone[7]. Other studies reported a prevalence of 85–95% of taste alteration associated with head and neck radiotherapy[7]. In addition, the persistence of dysgeusia was reported in 15% of overall cancer patients. In other, less severe cases, chemotherapy-related dysgeusia resolves within months, but even still, sometimes intervention can be indicated[30].
In the literature, the etiology, the pathophysiology, and the risk factors of taste alteration in cancer patients are still poorly studied. Nevertheless, it is now well established that taste alteration can largely affect the overall QoL of the patients, leading to energy and nutrient shortages, weight loss, malnutrition, depression, and significant morbidity[7]. Accordingly, we recommend further studies to focus more on the pathophysiology, the etiological factors, and the risk factors of taste alteration in cancer patients, which can, in turn, result in more adequate preventive and curative approaches.
In 2010, a systematic review of the Multinational Association of Supportive Care in Cancer and the International Society of Oral Oncology (MASCC/ISOO) concluded that among the present options, prophylactic use of zinc sulfate or amifostine had shown limited benefits, and that in order to minimize the symptoms of dysgeusia, nutritional counseling was used[31]. In 2020, a scoping review reported diverse alternative treatment modalities for the management of taste and smell complications in cancer patients[32]. The review endorsed that the currently available treatments are zinc supplements, polaprezinc mucosal protective agent, the use of radioprotectors (such as selenium and amifostine), the use of medical cannabis (specifically, delta-9-tetrahydrocannabinol), the use of photobiomodulation, the intake of dietary supplements (such as glutamine, lactoferrin, and fish oil), oral care, nutritional intervention, self-care, and the intake of fruits containing miraculin (such as the berry from West Africa) [32]. The review concluded that further studies must investigate in a larger and more methodological way the treatment modalities that have been proposed in the literature. In addition, a state-of-the-art review revealed that there is not yet an effective approach for the prevention or treatment of taste alteration in cancer patients. The review also concluded that the use of Marinol, Megestrol acetate, or Synsepalum dulcificum is among the most promising approaches but with no confirmation on its effectiveness[32]. Based on the present data, further studies are needed. In other words, and due to the current demands, it is of high significance to consider establishing an international protocol for the sake of managing taste alteration or dysgeusia (Figure 1).
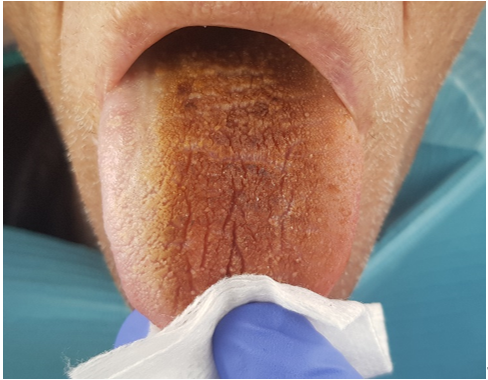
Figure 1. Dorsal aspect of the tongue after head and neck radiotherapy in a cancer patient.
4. Scale and Questionnaires for the Assessment of Taste Alterations
The use of a simple and easy-to-prepare procedure to assess taste is important in order to standardize the results of the studies, which will help researchers to compare and examine different studies.
Douglas et al. have suggested a detailed and well-described taste exam that consists of an oral cavity evaluation for potential disease and papilla identification. Furthermore, they have identified a psychophysical taste test that describes in detail the preparation of the testant and a taste questionnaire that the patient is asked to fill out after receiving the tastes, and after the collection of the genotype of the patient[33]. Additionally, the International Organization of Standards (ISO) has published a sensory analysis, including general guidelines for measuring flavor and taste (ISO 13301:2018)[34]. Furthermore, Kano T et al. developed a chemotherapy-induced taste alteration scale composed of 18 items, evaluated on a five-point type scale, and divided into three dimensions: quantitative changes in the perception of flavors, qualitative changes in the perception of flavors, and problems related to nutrition[35]. The “sip and spit” technique is simple to prepare and relatively reliable. After rinsing the patient’s mouth three times with room temperature distilled water, five solutions of the five basic tastes are distinctly tested. The solutions are 300 mM sucrose, 200 mM NaCl, 5 mM citric acid, 10 mM caffeine, and 200 mM monosodium glutamate (MSG). Correct responses are as follows: “sweet” for sucrose, “salty” for NaCl, “sour” for citric acid, “bitter” for caffeine, and “savory” for MSG. Further choices can be “none” or “metallic”. Perceived taste quality is identified by selecting one of the seven choices. Before and after sampling and expectorating each solution, the patient must rinse his mouth again. The score is assigned as 0 to 5 correct choices. If the patient fails to identify the correct taste, the score is 0, and if the answer was correct, 5. Before any examination and data collecting, the patient must be asked to stop eating and to drink only water at least one hour prior to testing.
5. Photobiomodulation Therapy and Its Possible Application for Taste Alteration
The fact that possible benefits might be obtained from the photobiomodulation therapy in the management of taste alteration can be explained by understanding the pathophysiology of taste alteration in cancer patients and relating it to the mechanism of action of photobiomodulation. Although the pathophysiology of dysgeusia is not fully understood, it is believed to be associated principally with the destruction of rapidly dividing taste bud cells, the destruction of olfactory receptor cells, and direct local neurological toxicity[32]. PBM therapy was reported to stimulate tissue regeneration, wound healing, and the reduction of inflammation[10][11]. Therefore, if applied with sufficient and adapted parameters and protocol, PBM can prevent the destruction of taste bud cells and olfactory receptor
s, as well as neurological toxicity[7][10][34]. In addition, if PBM is applied as a curative method for taste alteration, it can stimulate the healing and regeneration of these taste bud cells and olfactory receptors and can, therefore, stimulate the regeneration of the local neuronal complex. A recent study suggested that 650 and 808 nm light promotes intracellular Ca2+ elevation, regardless of cell type, but with different dynamics due to the specificities of Ca2+ regulation in neurons. This suggests that light-induced membrane depolarization is distinctly involved in the mechanism of Ca2+ influx. Ca2+ release from the endoplasmic reticulum, activated by reactive oxygen species generation, is considered as a possible light-dependent signaling pathway. [36].
When it comes to the use of PBM therapy for taste alterations, studies are still limited. In fact, it is necessary to have interventional clinical trials, meta-analyses, or systematic reviews on the use of PBM for taste alteration in cancer patients. However, in this matter, a pilot randomized clinical trial consisting of a placebo and a PBM group of patients with burning mouth sensations showed that the use of PBM was able to ameliorate the burning mouth sensation by inducing microcirculatory changes that persisted over a long period. An 800 nm wavelength diode laser was used twice a week for four consecutive weeks with 1200 Joules of energy, an irradiation time of 300 s, energy density of 50 J/cm2, 60 mW of continuous mode and irradiance of 180 mW/cm2. The improvement in the symptoms has been correlated to the size of the capillary diameter[37]. However, it is important to note that the exact energy density delivered per area was imprecisely described in the study and was significantly higher than the energy density cited in our study and of that recommended by Zecha et al. for the burning mouth sensation[7][10]. Whereas a case report and case series reported, respectively, that the use of PBM was able to treat dysgeusia and the burning mouth sensation in cancer patients[9][34].
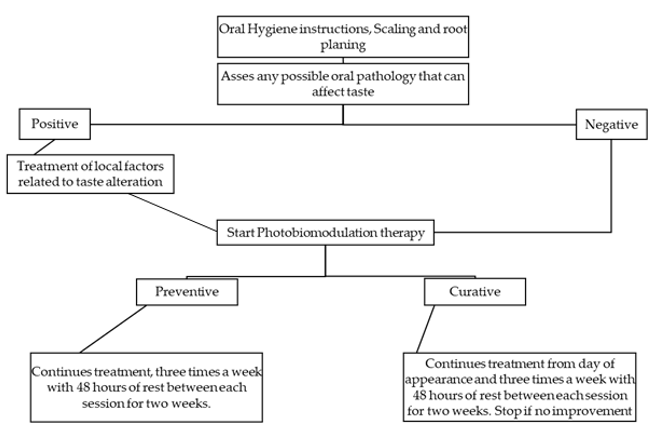
In 2016, a task force consisting of an international multidisciplinary panel of clinicians and researchers with expertise in the area of supportive care in cancer and photobiomodulation therapy formed and proposed in their study the use of specific PBM preventive and curative protocols and parameters for each of the oral complications of cancer patients[7][34]. The complications included in the study were oral mucositis, dermatitis, dysphagia, hyposalivation and xerostomia, taste alterations, trismus, soft tissue necrosis, and osteoradionecrosis, head and neck lymphedema, and voice and speech alterations. In order to standardize the protocol for future researchers that can be conducted on the use of PBM in the management of taste alteration, a protocol including proposed irradiation parameters was suggested. The suggested protocol was continuous treatment from the day a patient complains of taste alterations, for at least two or three times a week until symptoms improve. The use of wavelengths from 630–680 nm is suggested, with a power of 20 to 150 mW. The intra-oral irradiation is suggested for on the dorsal and lateral tongue at 3 J/cm2, and intra-orally on 10 points on the dorsum of the tongue.
Suggested Protocol
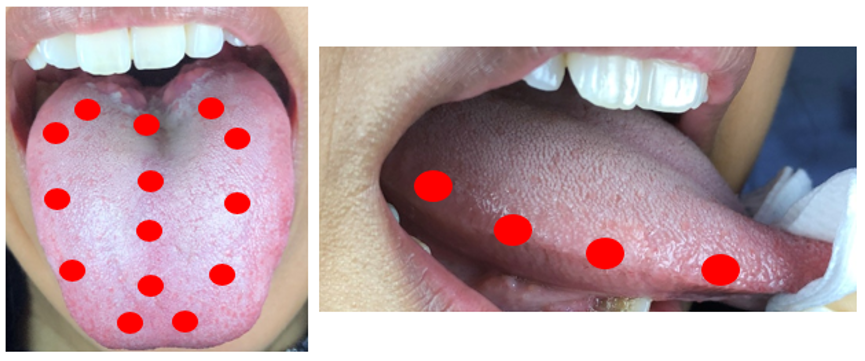
In this paragraph, a curative photobiomodulation protocol and parameters are suggested based on the evidence derived from the literature and experts’ opinions. The protocols were inspired largely by Zecha et al.’s study, with slight changes[7][10]. However, additional irradiation zones were suggested on the dorsum of the tongue, and four irradiation zones were suggested on the lateral surfaces (right and left) of the tongue due to the prominent presence of chemoreceptors of taste buds in this area. In addition, it is recommended to avoid possible overlaps during treatments (irradiation of the same area multiple times). The aim is to provide clinical guidance and to serve as a starting point for continuing research (Table 1, Figures 2 and 3).
Figure 2. Suggested protocol for the treatment of cancer therapy-induced taste alteration.
Table 1. Suggested photobiomodulation (PBM) parameters for the preventive and curative management of taste alteration.
|
Complication |
Treatment Protocol |
Treatment Area |
PBM Parameters |
|
Preventive |
Continuous treatment from the T (0), three times a week with 48 h of rest between each session for two weeks. |
A total of 14 points on the dorsum of the tongue and a total of 3 points on the lateral bord of the tongue. |
Diode laser wavelength of 600–810 nm. E = 2 J on each point, T = 10–40 s, P = 20–400 mW, one irradiation per treated surface of 1 cm2, continuous mode, contact mode. |
|
Curative |
Continuous treatment from day of appearance and three times a week with 48 h of rest between each session for two weeks. Stop if no improvement. |
A total of 14 points on the dorsum of the tongue and a total of 3 points on the lateral bord of the tongue. |
Diode laser wavelength of 600–810 nm. E = 4 J on each point, T = 10–40 s, P = 20–400 mW, one irradiation per treated surface of 1 cm2, continuous mode, contact mode. |
(T0) = First day of cancer treatment. E = energy (J); T = Time (seconds); P = Power (mW).
Figure 3. Treatment areas of PBM for the treatment of taste alteration.
6. Concluding Remarks and Discussion
PBM may be of interest in the management of taste alteration in cancer patients by playing a possible role in the recovery of altered taste bud cells and by enhancing salivation, which may have a significant contribution to general taste alteration. It is also important to note that there is still a lack of understanding of the exact effect and mechanism of action of PBM. The optimal dose delivered to the targeted taste buds, the ideal wavelength, and the irradiation parameters for each case are still primordial factors that need to be well understood.
Further studies and investigations are needed on the pathophysiology of taste alteration following radiotherapy and/or chemotherapy in cancer patients. In addition, a gold standard protocol for PBM therapy is needed for the treatment of taste alteration in cancer patients.
References
- Hadley, ; Orlandi, R.R.; Fong, K.J. Basic anatomy and physiology of olfaction and taste. Otolaryngol. Clin. N. Am. 2004, 37, 1115–1126.
- Breslin, A.; Huang, L. Human taste: Peripheral anatomy, taste transduction, and coding. Tast. Smell 2006, 63, 152–190.
- Drareni, ; Anestis, D.; Agnes, G.; Martine, L.; Pierre-Jean, S.; Moustafa, B. Relationship between food behavior and taste and smell alterations in cancer patients undergoing chemotherapy: A structured review. In Seminars in Oncology; WB Saunders: Philadelphia, PA, USA; 2019; Volume 46, pp. 160–172.
- Risso, ; Drayna, D.; Morini, G. Alteration, Reduction and Taste Loss: Main Causes and Potential Implications on Dietary Habits. Nutrients 2020, 12, 3284.
- Kiss, ; Symons, K.; Hewitt, J.; Davis, H.; Ting, C.; Lee, A.; Boltong, A.; Tucker, R.M.; Tan, S.Y. Taste Function in Adults Undergoing Cancer Radiotherapy or Chemotherapy, and Implications for Nutrition Management: A Systematic Review. J. Acad. Nutr. Diet. 2020, 15, 2212–2672.
- Hovan, J.; Williams, P.M.; Stevenson-Moore, P.; Wahlin, Y.B.; Ohrn, K.E.; Elting, L.S.; Spijkervet, F.K.; Brennan, M.T. Dysgeusia Section, Oral Care Study Group, Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). Support Care Cancer 2010, 18, 1081–7.
- Zecha, A.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M.; Genot, M.T.; et al. Low-level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: Part 2: Proposed applications and treatment protocols. Support Care Cancer 2016, 24, 2793–2805.
- Epstein, B.; Barasch, A. Taste disorders in cancer patients: Pathogenesis, and approach to assessment and management. Oral Oncol. 2010, 46, 77–81.
- El Mobadder, ; Farhat, F.; El Mobadder, W.; Nammour, S. Photobiomodulation Therapy in the Treatment of Oral Mucositis, Dysgeusia and Oral Dryness as Side-Effects of Head and Neck Radiotherapy in a Cancer Patient: A Case Report. Dent. J. 2018, 6, 64.
- Zecha, A.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Sonis, S.T.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M.; et al. Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: Part 1: Mechanisms of action, dosimetric, and safety considerations. Supportive Care Cancer 2016, 24, 2781–2792.
- Bensadoun, J.; Epstein, J.B.; Nair, R.G.; Barasch, A.; Raber-Durlacher, J.E.; Migliorati, C.; Genot-Klastersky, M.T.; Treister, N.; Arany, P.; Lodewijckx, J.; et al. World Association for Laser Therapy (WALT). Safety and efficacy of photobiomodulation therapy in oncology: A systematic review. Cancer Med. 2020, 9, 8279–8300.
- Elad, ; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 1, 4423–4431.
- Serrage, ; Heiskanen, V.; Palin, W.M.; Cooper, P.R.; Milward, M.R.; Hadis, M.; Hamblin, M. R. Under the spotlight: Mechanisms of photobiomodulation concentrating on blue and green light. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2019, 18, 1877–1909.
- Karu, I. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. Iubmb Life 2010, 62, 607–10.
- Chung, ; Dai, T.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533.
- Bjordal, M. Low level laser therapy (LLLT) and World Association for Laser Therapy (WALT) dosage recommendations. Photomed. Laser Surg. 2012, 30, 61–62.
- Jenkins, A.; Carroll, J.D. How to report low-level laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomed. Laser Surg. 2011, 29, 785–787.
- Guirro, R.; Weis, L.C. Radiant power determination of low-level laser therapy equipment and characterization of its clinical use procedures. Photomed Laser Surg. 2009, 27, 633–639.
- Sembulingam, ; Sembulingam, P. Essentials of Medical Physiology; Jaypee Brothers Medical Publishers (P) Ltd. New Delhi, India,: 2012.
- Breslin, A. An evolutionary perspective on food and human taste. Curr. Biol. 2013, 6, 409–418.
- Vincis, ; Fontanini, A. Central taste anatomy and physiology. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 164, pp. 187–204.
- Feeney, L.; Hayes, J.E. Regional Differences in Suprathreshold Intensity for Bitter and Umami Stimuli. Chemosens. Percept. 2014, 7, 147–157.
- Colvin, L.; Pullicin, A.J.; Lim, J. Regional Differences in Taste Responsiveness: Effect of Stimulus and Tasting Mode. Chem. Senses 2018, 43, 645–653.
- Higgins, J.; Hayes, J.E. Regional Variation of Bitter Taste and Aftertaste in Humans. Chem. Senses 2019, 44, 721–732.
- Sandow, L.; Hejrat-Yazdi, M.; Heft, M.W. Taste loss and recovery following radiation therapy. J. Dent. Res. 2006, 85, 608–11.
- Epstein, B.; Smutzer, G.; Doty, R.L. Understanding the impact of taste changes in oncology care. Support Care Cancer 2016, 24, 1917–1931.
- Kamprad, ; Ranft, D.; Weber, A.; Hildebrandt, G. Functional changes of the gustatory organ caused by local radiation exposure during radiotherapy of the head-and-neck region. Strahlenther. Onkol. 2008, 184, 157–62.
- Peregrin, Improving taste sensation in patients who have undergone chemotherapy or radiation therapy. J. Am. Diet. Assoc. 2006, 106, 1536–1540.
- Mese, ; Matsuo, R. Salivary secretion, taste and hyposalivation. J. Oral Rehabil. 2007, 34, 711–723.
- Gamper, M.; Zabernigg, A.; Wintner, L.M.; Giesinger, J.M.; Oberguggenberger, A.; Kemmler, G.; Sperner-Unterweger, B.; Holzner, B. Coming to your senses: Detecting taste and smell alterations in chemotherapy patients. A systematic review. J. Pain Symptom Manag. 2012, 44, 880–895.
- Hovan, J., Williams, P.M., Stevenson-Moore, P., Wahlin, Y.B., Ohrn, K.E., Elting, L.S., Spijkervet, F.K.; Brennan, M.T. A systematic review of dysgeusia induced by cancer therapies. Supportive Care Cancer 2010, 18, 1081–1087.
- Sevryugin, ; Kasvis, P.; Vigano, M.; Vigano, A. Taste and smell disturbances in cancer patients: A scoping review of available treatments. Supportive Care Cancer 2021, Jan;29(1):49-66.
- Douglas, E.; Mansfield, C.J.; Arayata, C.J.; Cowart, B.J.; Colquitt, L.R.; Maina, I.W.; Blasetti, M.T.; Cohen, N.A.; Reed, D.R. Taste Exam: A Brief and Validated Test. JoVE (J. Vis. Exp.) 2018, 138, 56705.
- El Mobadder, ; Farhat, F.; El Mobadder, W.; Nammour, S. Photobiomodulation Therapy in the Treatment of Oral Mucositis, Dysphagia, Oral Dryness, Taste Alteration, and Burning Mouth Sensation Due to Cancer Therapy: A Case Series. Int. J. Environ. Res. Public Health 2019, 16, 4505.
- Kano, ; Kanda, K. March. Development and validation of a chemotherapy-induced taste alteration scale. Oncol. Nurs. Forum 2013, 40, 79–85.
- Golovynska, ; Golovynskyi, S.; Stepanov, Y.V.; Stepanova, L.I.; Qu, J.; Ohulchanskyy, T.Y. Red and near-infrared light evokes Ca2+ influx, endoplasmic reticulum release and membrane depolarization in neurons and cancer cells. J. Photochem. Photobiol. B 2020, 214, 112088.
- Scardina, G.A.; Casella, S.; Bilello, G.; Messina, P. Photobiomodulation Therapy in the Management of Burning Mouth Syndrome: Morphological Variations in the Capillary Bed. Dent. J. 2020, 8, 99.