Hard tissues, e.g., bone, are mechanically stiff and, most typically, mineralized. To design scaffolds for hard tissue regeneration, mechanical, physico-chemical and biological cues must align with those found in the natural tissue. Combining these aspects poses challenges for material and construct design. They can be fulfilled in top-down tissue engineering or bottom up biofabrication approaches upon employing biomaterials e.g. silk.
- Hard Tissue Engineering
- Biomineralization
- Composite Material
- Biofabrication
- Biomaterial
- Mechanics
- Physico-Chemistry
- Biological Binding Sites
- Introduction
The development of hard tissue in the human body is a process of mineral formation by cellular metabolism, named biomineralization, yielding support structures of the skeleton and neighboring tissues such as tendon and cartilage or functional tissues such as teeth [1]. There are several different mineralization pathways, but they are not yet fully explored [2]. Generally, mineral formation in tissues needs to be highly controlled to prevent local over-mineralization, which could be pathogenic [2]. The high process control of biomineralization is provided by tissue-specific cells and biopolymers such as proteins, which are templating and nucleating mineral formation [3]. Therefore, biogenic crystals often exhibit a different morphology than their geogenic counterpart [4].
Tissue-specific cells are taking a crucial role in biomineralization as they trigger mineral nucleation and growth upon secretion of so-called non-collagenous proteins [2,5]. The main proteinous material (90 wt.%) of hard tissues is collagen type I as flexible filler in this composite material, while the non-collagenous proteins cover the remaining 10 wt.% [2]. Collagen is not mineralized on its own, but collagen fibrils can interact with non-collagenous proteins, which induce mineralization from saturated media at the gap regions of the stacked triple-helical collagen fibrils [2,5]. The phosphorylated, non-collagenous proteins of the so-called SIBLING family (Small Integrin-Binding Ligand, N-Linked Glycoprotein) include bone sialoprotein and osteopontin in bone-related tissues, whereas in teeth dentin and cementum, dentin matrix protein 1 and dentin phosphoryn are present. These proteins provide two functions, as on the one hand, they can bind at specific locations to the structural collagen scaffold and on the other hand, they can bind ions due to their, in most cases, highly charged nature with repetitive motifs of glutamic or aspartic acid residues [5]. This local charge density allows to accumulate mineral ions and, thereby, to initiate crystal nucleation, when the ion density reaches a critical concentration, which then triggers the further mineralization processes in mineralized tissues, such as bone, teeth, cartilage and tendon [5].
Further, mineralization is driven by tissue-related osteoblasts (in bone and tendon), odontoblasts (in teeth) and chondrocytes (in cartilage) upon the accumulation of ions from the surrounding environment in mostly separated membrane vesicles [6]. With ongoing mineralization, the extracellular matrix around these cells densifies, and nutrients and oxygen are increasingly provided only passively by diffusion until easy nutrient supply is finally prevented. In the case of bone and neighboring tissue, osteoblasts differentiate into osteocytes [1]. Osteoclasts, on the other hand, are constantly remodeling fully mineralized tissue to guarantee healthy and reconstructed bone [7].
For traditional and engineering approaches to reconstruct hard tissues, natural processes have to be understood. Further, as bone represents the most abundant fully mineralized tissue, a majority of tissue engineering approaches focus on respective reconstructive solutions. Bone defects such as fractures easily occur, for example due to critical non-physiologically high loads. Shortly after fracture, inflammatory responses are initiated at the defect site, followed by a cell-induced regeneration cascade for initial callus formation, which is then remodeled to form new bone. With progressing age, bones become increasingly brittle due to changes in the cellular metabolism of osteoblast cells, which is indicated by 10–40 times lower strain rates until breakage. One possible reason might be remodeling cycles, which affect the mineral phase and allow more micro-cracking, finally leading to bone failure [8]. Once fractured, bone defects can be detected by x-raying of the defect site. New techniques such as ultrasonography for detecting bone fractures are more sensitive than classical radiographs, which are typically used to trace fractures of long bones. Sonographic methods provide the advantage of no radiation exposure, lower cost and wider availability in non-hospitals. A study among German general practitioners showed that most articulated sono-methods are inferior to classical x-ray [9]. In clinical procedures, the defect site is often bridged and stabilized with bone platelets or screws to stabilize material in place during the regeneration process [10]. In order to further support bone healing or large defects with bone loss, hard tissue engineering methods are increasingly used. In contrast to the self-regenerating ability of bone tissue, other mineralized tissues rely more on artificial replacement than supportive healing.
- Tissue Engineering Approaches
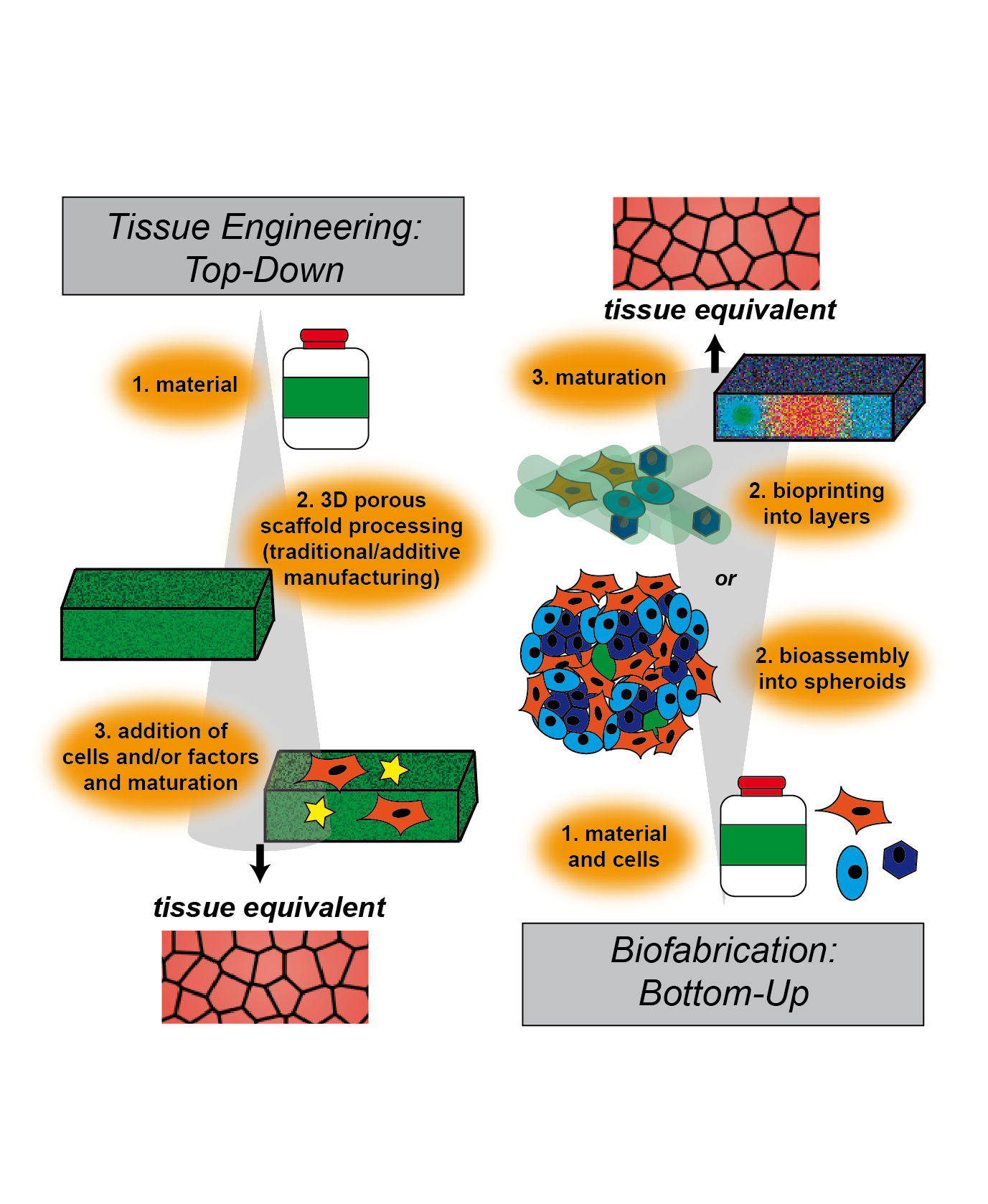
Defined in 1993, tissue engineering is the combination of principles of engineering and life sciences with the goal of developing biological substitutes that are able to restore, maintain or improve biological tissue function or a whole organ [11,12]. While one part of this interdisciplinary field deals with the generation of 3D models for the development of therapies, its main potential lies in regenerative medicine. With the goal of replacing tissues and organs damaged by trauma, disease or age, the ever-extending field of TERM (tissue engineering and regenerative medicine) includes basic and advanced cell and stem cell biology, scaffold material research and various fabrication and processing techniques [13,14]. The classical TERM approach to overcome drawbacks of autografts or allografts consists of scaffold-based top-down strategies (Figure 1). Such engineered tissues are typically created by manufacturing biodegradable polymeric scaffolds on which cells are seeded. During cultivation, and in some cases stimulated by perfusion, growth factors or mechanical cues, cells dynamically remodel and replace the scaffold through degradation and new extracellular matrix (ECM) deposition [15]. Traditionally, three-dimensional scaffolds are manufactured by employing techniques such as freeze-drying, leaching of particles or salt, chemical or gas foaming and thermally induced phase separation [16–19]. While these processes allow partial control over the scaffold properties, there are severe restrictions to generate precise micro-architectures, including pore size, geometry and connectivity.
With the rise of additive manufacturing, more techniques are available to create scaffolds for tissue engineering, overcoming previous restrictions. With an emphasis on the regeneration of bone tissue, four major layer-by-layer methods have been reviewed recently by Madrid et al. [20]. A variety of natural and synthetic polymers, as well as ceramics and bioceramics and even metals can be processed using stereolithography (SLA), selective laser sintering (SLS), fused deposition modeling (FDM) and three-dimensional printing (3DP). These techniques generally allow for more accurate scaffolds with better resolution. The specific processes, including laser and heat treatment, nevertheless tremendously restrict the choice of material [20]. Since additive manufacturing is based on computer-aided-design, structures that are more sophisticated can be created, including patient-specific scaffolds with the help of computer tomography. While these top-down approaches allow for good reproduction of the macroscopic structures of desired tissues and organs, the complexity, micro-arrangement and heterogeneity of natural tissues, including different cell types and materials, is far beyond what is found in such artificial acellular scaffolds [15,21].
To overcome this limitation, a multitude of bottom-up approaches has been developed in recent years. In contrast to traditional top-down approaches, where first the scaffold is produced, followed by seeding with cells, in bottom-up approaches, cells are used from the very beginning in combination with materials to build up tissue constructs step-by-step (i.e., bottom-up) (Figure 1). Biocompatible materials in various morphologies, like particles, one-dimensional fibers, two-dimensional films and three-dimensional hydrogels, have been used, alongside cells, as building blocks to generate assemblies at the nano- or micro-scale. Further self- or directed-assembly leads to engineered macroscopic three-dimensional tissue constructs. A comprehensive review examining these advanced bottom-up approaches has recently been published by Gaspar et al. [21,22].
Figure 1. Schematic illustration of engineering approaches to fabricate tissue. In top-down strategies, a scaffold is produced, followed by cell seeding and/or addition of factors for cellular stimulation. This technique is called tissue engineering. Bottom-up approaches use cells and raw materials simultaneously to build larger constructs, which are then maturated. This technique is called biofabrication.
Benefits of these strategies include the involvement of cells in the development of the tissue right from the beginning, as well as the possibility to generate constructs made from different types of assemblies, leading to various cell types and materials organized hierarchically within the resulting construct. Different assemblies can be divided into two main categories, mostly scaffold-free cell-rich and cell-biomaterial assemblies. Adhesive surfaces, possibly in combination with functionalized and/or non-adhesive surfaces, are used to generate monolayer cell sheets by cultivation and subsequent detachment of the grown layer. Stacking, rolling and folding of these monolayer sheets is the basis to create combined three-dimensional assemblies, including multicellular and pre-vascularized constructs [23,24]. By using cellular spheroids, often made of mesenchymal stem cells, as scaffold-free building blocks, processes like cell-cell and cell‑ECM interactions, differentiation and fusion are recapitulated [25,26]. In addition, genetic or chemical engineering of the cell surface allows control over cellular behavior and assembly into higher-order structures [27,28]. Inclusion of biological materials is a crucial part of bottom-up tissue engineering strategies, such as the addition of biocompatible layers within cellular sheets, functionalized with nucleic acids, viruses, enzymes and structural proteins, as well as peptides and polymers. To increase structural assembly within cellular spheroids or hydrogels, fibers and particles can be incorporated. These materials can add structural support and guidance, promote and/or control the assembly of building blocks and stimulate cellular behavior in general [21,29,30]. For example, a silk fibroin derived hydrogel was used as a scaffold for articular cartilage tissue engineering, and integrated poly(lactid-co-glycolid) nanoparticles were used to simultaneously deliver two growth factors, resulting in beneficial effects on proliferation and differentiation of dental pulp stem cells [31]. On the way to tissue or organ replacement, such multicellular and multimaterial assemblies are used to generate vascularized multicomponent constructs or spatially organized multiblock hydrogels [21].
In the context of advanced bottom-up tissue engineering approaches, a new field called biofabrication has been reviewed recently by Groll et al. [32]. Biofabrication mainly, but not solely, uses additive manufacturing techniques to process bottom-up building blocks into hierarchically structured cell-biomaterial constructs. Biofabrication describes the automated generation of biologically functional constructs through bioprinting, meaning the direct spatial arrangement of cells, materials and/or factors, and through the automated assembly of cell-containing building blocks, so-called bioassembly. In both cases, in vitro maturation and or fusion of the products is a crucial step before obtaining a tissue equivalent for implantation or pharmaceutical screening [32]. Relevant technologies within biofabrication have been recently reviewed by Moroni et al. [33]. With the possibility of simultaneous deposition of cells and material in an additive manufacturing process, bioplotting, ink-jet bioprinting and valve-jet bioprinting are major biofabrication tools for bottom-up tissue engineering and regenerative medicine. Formulations of materials, cells and biological molecules, so-called bioinks, are processed using these technologies. Bioplotting, also called robotic dispensing or extrusion bioprinting, dispenses continuous filaments of hydrogel materials or bioinks through a nozzle (piston-, screw-, or pneumatic-driven). Droplets are ejected over a nozzle head, controlled either by piezo- and thermal-actuators (ink-jet) or by solenoid micro-valves (valve-jet) [32,33].
All approaches, whether they include manufacturing a scaffold followed by cell-seeding or bioprinting/bioassembly, have strict requirements on the used material. Physical and mechanical properties need to be suitable for processing using the respective technology on the one hand and ensure cellular survival and proliferation on the other. The material also plays an important role in guiding specific cellular development and maturation, for example, by surface functionalization, the inclusion of biological molecules or the tuning of degradation behavior. With the goal of implantation of constructs, biocompatibility, meaning the performance of intended purpose without evoking an immune response, is absolutely required and can be enhanced e.g. upon introduction of nanoparticles [34-36]. Due to their inherent biological and chemical similarities to native tissue, natural polymers, natural polymer-based composites and bioceramics are of great interest for tissue engineering applications. Due to the high load-bearing requirement, hard tissue engineering approaches so far mainly focus on top-down strategies using porous scaffolds for cell seeding [37,38].
- Hard Tissue Engineering
3.1. State of the Art
After diagnosis of a bone defect, the respective site is commonly deprived from extensive movement as both bone sides need to reconnect during regeneration in a correct manner, otherwise malfunction might be the result of improper healing. The origin of the cells, which are taking part in bone repair, were found to influence the healing progress. The cells present in bone encompass, for example, stem cells during bone healing or endothelial cells building vasculature, but also pre-osteoblasts, which differentiate into osteocytes during bone formation and maturation as described above. Osteoclasts are undertaking the function of degradation, which is a continuously ongoing process to maintain healthy bone and allow for expansion of the skeleton during the development of children [39]. When artificially delivered into bone defects, neural crest-derived frontal bone and mesoderm-derived parietal bone cells from newborn rats were found to exhibit both similar bone regeneration ability, although the mesodermal cells showed a potentially higher bone regeneration efficiency in vitro [40]. MC3T3 E1 pre-osteoblast cells were posed in hydroxyapatite microcracks similar to bone fractures and found to underlie initial apoptosis at a region of 200 nm around the cracks [41]. Besides fixation, flexoelectricity, meaning the ability to generate electricity under pressure, was found crucial for bone healing [41]. Exposed to strain such as physical activity during bone healing, bone regeneration was increased, and so rehabilitation measures actively contributed to tissue regeneration [42]. With near-infrared fluorescent probes,[43] bone repair could be imaged concerningin vitro differentiation of human mesenchymal stem cells into osteoblasts. A cyclic peptide coupled with a fluorophore was used to bind to α5β1 integrin as an osteoblast-specific marker. A second probe was coupled with the drug pamidronate to a fluorescent gold nanocluster, where the drug bound specifically to hydroxyapatite and allowed for monitoring osteogenesis [43].
Loss of bone material due to cancer or other pathogenic relations such as osteoporosis is often not recovered spontaneously and needs tissue replacement. Autologous bone grafts are still considered as the gold standard transplant due to facilitated integration at the defect site. As concerns about donor availability, healing and disease transmission arise, artificial bone substitutes become increasingly attractive to overcome these obstacles [39]. Therefore, titanium implants are state of the art as they are biologically inert materials, which offer high load transmission. Unfortunately, these foreign body materials are rarely fully integrated into the surrounding tissue and might become loose; therefore, surgical rearrangement might be necessary. One major reason for this issue is a bacterial infection, especially concerning dental implants with extensive biofilm formation [34]. To improve integration, for example titanium alloy (Ti6Al4V) implants with TiO2 nanotubes were coated with silk fibroin, which was found to enhance osteoconductive and osteogenic properties in case of bone implant performance [44]. Bone, cell and implant interaction was found to be enhanced for MG63 bone cells and human mesenchymal stem cells, which is beneficial for implant applicability. Biomimetic minerals for hard tissue engineering, which enhance osseointegration, can rely on biosimilars such as calcium sulfate or phosphate ceramics as synthetic and hydroxyapatite as a naturally occurring form of bone mineral [39]. Building scaffolds out of these materials can be realized upon melting and fusing individual ceramic particles using laser sintering at temperatures above 1000 °C [45–47]. Utilizing this rapid prototyping technique, also polymeric carrier materials can be fused at lower temperatures (about 70–200 °C) whilst molding and binding ceramic particles into bionanocomposites and simultaneously removing the binder [48,49]. With such polymeric binders, 3D extrusion and additional sintering of the composite materials is possible, yielding solely the remaining solid ceramic structures (Figure 2) [50,51].
Further, injectable calcium phosphate cements including ceramics and a curing agent were invented by Brown and Cho in the 1980ies to fill dental cavities in the first place [52]. As state of the art, synthetic polymeric materials are widely used as matrix materials in hard tissue engineering, however, they often cannot complement features of biomaterials such as non-toxic degradation products and bioactive surfaces for cell adhesion [53].
3.2. Design Criteria and Challenges
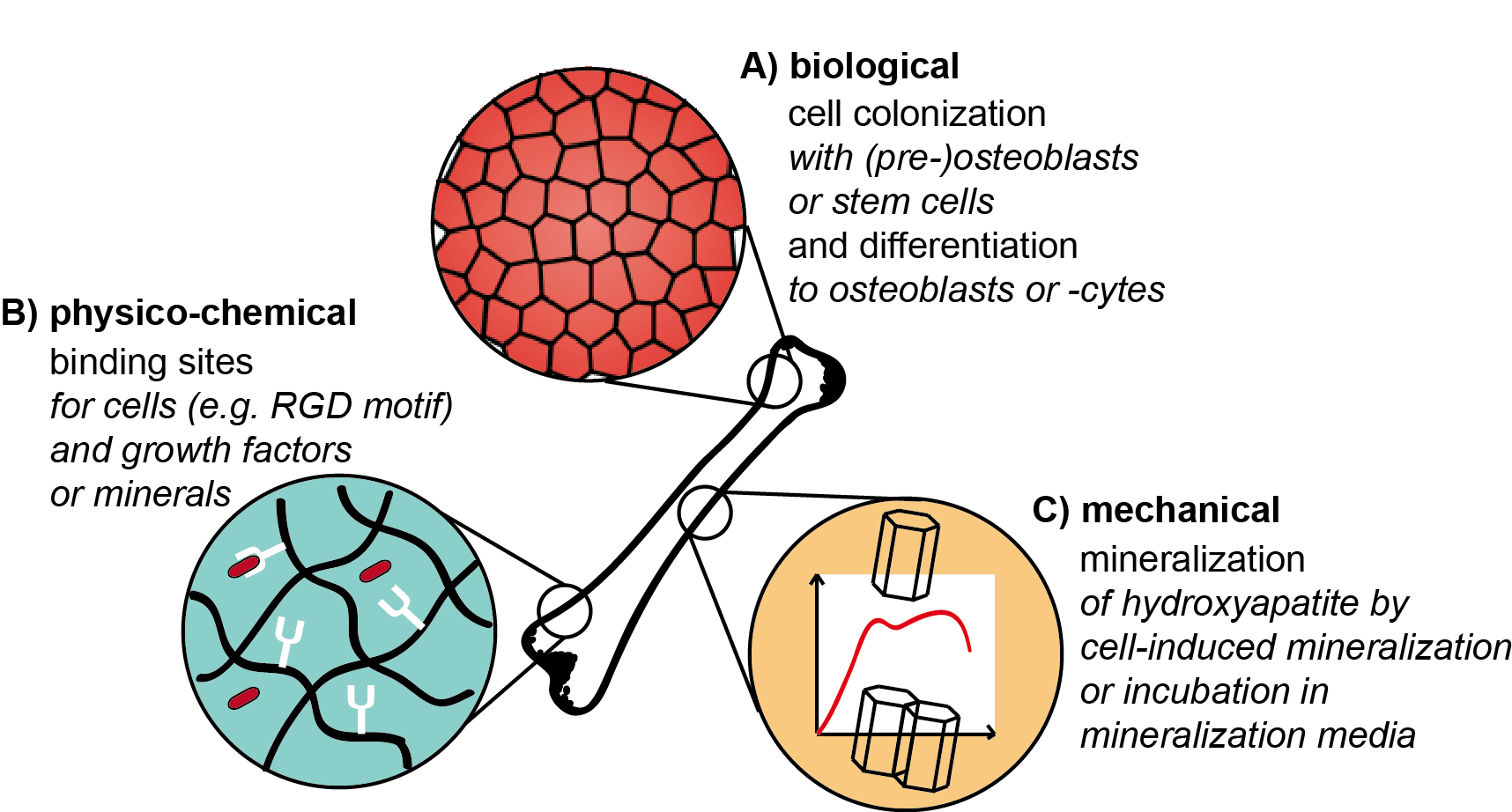
It is important that various design criteria and factors have to be taken into account in tissue engineering approaches to fulfill the requirements of a successful tissue engineering construct (Figure 2). In the case of hard tissues, besides biological and physico-chemical cues, also the appropriate mechanics play an important role. In the following, these aspects are discussed in more detail and illuminated why they can be challenging during scaffold preparation.
Figure 2. Illustration of biological, mechanical and physico-chemical factors of scaffold materials relevant for hard tissue engineering. (A) Media, growth factors, etc. are needed for tissue-specific cell colonization and differentiation on artificial scaffolds. (B) Materials/scaffolds have to provide binding sites for cells, factors and minerals. (C) Biomineralization is necessary to gain composite materials with adopted mechanical properties (such as stiffness, etc.).
Concerning the mechanical design, it has to be taken into account that mature bone has compressive strengths in the order of up to 20 GPa [54], whereas they are far lower for immature bone, as the mineralization process is still ongoing [55,56]. However, not only high strength but also flexibility must be provided. Therefore, mostly brittle materials are not suitable for bone regeneration applications, as the risk of failure is high [8]. It can be challenging to combine high load-bearing materials with high flexibility, but these mechanical requirements can be fulfilled in biomaterial matrices applying reinforcing filler materials such as ceramic particles into composite materials. To gain homogenous mineralization, it is important that filler and matrix material interact well with each other to avoid phase separation, which is an additional criterion. Practical hints can be found when taking a closer look at the natural blueprint: Bone is a composite material [57] with collagen fibrils (20–30 wt.%) and ceramic particles made of hydroxyapatite (60–70 wt.%) [2]. Besides composite materials, biomineralization of protein precursor materials can be triggered in vitro upon immersion in mineralization agents forming calcium phosphate species. These can for example be single aqueous salt solutions, which are subsequently applied to the materials [58–60]. More complex mineralization is provided by Simulated Body Fluid, a model solution at pH 7.4, which was designed to simulate mineralization processes found during bone formation. Its ion composition and concentration are proximately close to human blood plasma [61].
Tailoring mechanical properties upon controlled mineralization is highly interconnected with the scaffold’s biological function and vice versa. During mineralization of tissue, cells play an important role as they secrete non-collagenous proteins with highly located charge [2]. Especially SIBLING proteins [5] are to be mentioned among others, as they coordinate nucleation, growth and inhibition phase during mineral formation as they accumulate ions from the surrounding intestinal fluids [62]. Further, hydroxyapatite precursor phases can be accumulated in cell membrane-bound vesicles and released at mineralization sites [6]. As a result, tissue-specific cell colonization is an additional design cue to mimic natural tissue in engineered constructs. Its respective challenge is posed not only by cell adhesion to the surface or in the construct but also to trigger osteoblast lineage in osteoblast precursor cells or stem cells. Biomineralization and osteogenic differentiation were found to be highly dependent on matrix stiffness [63]. 2D surfaces of different controllable substrate stiffness showed the best results for medium stiffness (50–100 kPa), as mineralization was completed after three weeks. Osteoblast differentiation was directly related to the formed mineral layer and only indirectly regulated by matrix stiffness [63]. The release of ions from the material, which is sensed by cells, can also lead to differentiation responses. One example for such materials is 45S5 Bioglass embedded in silk fibroin/gelatine scaffolds [64]. The bioglass composition comprises SiO2, CaO, Na2O and P2O5, and the ion release profile triggers osteogenic cell differentiation [65]. For this functionalization, it is important to control the osmotic balance of the media for cell survival.
Moreover, not only mechanical but also physico-chemical properties of the scaffold can lead to desired cell differentiation. The design of such cues can be related to binding sites for cells, growth factors or minerals. Besides cell-specific binding motifs [66], the integrin binding peptide motif arginyl glycyl aspartic acid (RGD) [67] is universally applied. The incorporation of this motif can be a challenge when it is not intrinsically provided by the biomaterial. This can be solved upon genetic engineering of proteins used in the material or chemical coupling of the motif to the material [68]. Related to the natural tissue, growth factors such as the most important one, the Transforming Growth Factor beta (TGF-beta), as well as bone morphogenetic proteins are agreed to have a beneficial impact on the success of hard tissue engineering scaffolds [69]. The factors can be delivered via the construct and trigger stem cells towards osteo-differentiation [70]. As a challenge, their concentration must be maintained [69] at levels confirmed to be active (nM) during cell cultivation by specific binding, otherwise, scaffolds become depleted fast by diffusion. Binding sites for ions were discussed above to be provided by non-collagenous proteins with located charges. Mimics of these proteins can be designed and incorporated into the scaffold. However, the preparation of hybrid proteins from silk and non-collagenous proteins can be challenging to gain functional mineral binding sites [71,72].
Taking all these complex requirements into regard, scaffolds must comply not only with mechanical but also physico-chemical and biological demands to build a successful hard tissue engineering construct. One crucial role plays the material choice. The named requirements can be met, for example, with synthetic or natural materials [14]. However, synthetic materials pose the risk of toxic degradation products during tissue regeneration, and their biocompatibility is limited [73]. Naturally derived materials avoid these obstacles. Further, they naturally can provide biological and/or mineral binding sites. However, disease transmission from donor animal sources and material heterogeneity must be avoided. Collagen and gelatin are common materials due to their native occurrence in bone and the presence of biomineralization nucleation sites. Moreover, bone takes a long time to develop, and the collagen will often degrade before it can be remodeled. Among artificial natural biomaterials, silk appears to be an attractive material, as it provides non-toxicity and biodegradability. Further, silk proteins can be produced biotechnologically, modified and processed into a variety of morphologies [73]. The upcoming sections will shed light on how silk materials can be used, for example, as matrix materials for bone tissue engineering.
References
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic Systems for Hydroxyapatite Mineralization Inspired by Bone and Enamel. Rev. 2008, 108, 4754–4783, doi:10.1021/cr8004422.
- Beniash, E. Biominerals—hierarchical nanocomposites: The example of bone. WIRES Nanomed. Nanobi. 2011, 3, 47–69, doi:10.1002/wnan.105.
- Gower, L.B. Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and Its Role in Biomineralization. Rev. 2008, 108, 4551–4627, doi:10.1021/cr800443h.
- Weiner, S.; Addadi, L. Crystallization Pathways in Biomineralization. In Annual Review of Materials Research; Clarke, D.R., Fratzl, P., Eds; Annual Reviews: Palo Alto, CA, USA, 2011; Volume 41, pp. 21-40.
- George, A.; Veis, A. Phosphorylated Proteins and Control over Apatite Nucleation, Crystal Growth, and Inhibition. Rev. 2008, 108, 4670–4693, doi:10.1021/cr0782729.
- Anderson, H.C. Matrix vesicles and calcification. Rheumatol. Rep. 2003, 5, 222–226, doi:10.1007/s11926-003-0071-z.
- Zhang, T.; Lin, S.; Shao, X.; Zhang, Q.; Xue, C.; Zhang, S.; Lin, Y.; Zhu, B.; Cai, X. Effect of matrix stiffness on osteoblast functionalization. Cell Prolif. 2017, 50, e12338, doi:10.1111/cpr.12338.
- Zioupos, P.; Kirchner, H.O.; Peterlik, H. Ageing bone fractures: The case of a ductile to brittle transition that shifts with age. Bone 2020, 131, 115176, doi:10.1016/j.bone.2019.115176.
- Schmid, G.L.; Kühnast, B.; Heise, M.; Deutsch, T.; Frese, T. Ultrasonography in assessing suspected bone fractures: A cross-sectional survey amongst German general practitioners. BMC Fam. Prac. 2020, 21, 1–7, doi:10.1186/s12875-020-1078-5.
- Li, J.; Qin, L.; Yang, K.; Ma, Z.; Wang, Y.; Cheng, L.; Zhao, D.W. Materials evolution of bone plates for internal fixation of bone fractures: A review. Mater. Sci. Technol. 2020, 36, 190–208, doi:10.1016/j.jmst.2019.07.024.
- Langer, R.; Vacanti, J. Tissue engineering. Science 1993, 260, 920–926.
- Hoffman, T.; Khademhosseini, A.; Langer, R. Chasing the Paradigm: Clinical Translation of 25 Years of Tissue Engineering. Tissue Eng. Part A 2019, 25, 679–687, doi:10.1089/ten.tea.2019.0032.
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Natl. Acad. Sci. USA 2015, 112, 14452–14459, doi:10.1073/pnas.1508520112.
- Ramos, T.; Moroni, L. Tissue Engineering and Regenerative Medicine 2019: The Role of Biofabrication—A Year in Review. Tissue Eng. Part C: Methods 2019, 26, 91–106, doi:10.1089/ten.tec.2019.0344.
- Nichol, J.W.; Khademhosseini, A. Modular tissue engineering: Engineering biological tissues from the bottom up. Soft Matter 2009, 5, 1312–1319, doi:10.1039/b814285h.
- Chen, P.; Zhou, Z.; Liu, W.; Zhao, Y.; Huang, T.; Li, X.; Duan, J.; Fang, J. Preparation and Characterization of Poly(L-lactide-co-glycolide-co-ε-caprolactone) Scaffolds by Thermally Induced Phase Separation. Macromol. Sci. Part B 2020, 59, 427–439, doi:10.1080/00222348.2020.1735136.
- Januariyasa, I.K.; Yusuf, Y. Porous carbonated hydroxyapatite-based scaffold using simple gas foaming method. Asian Ceram. Soc. 2020, 8, 634–641, doi:10.1080/21870764.2020.1770938.
- Judawisastra, H.; Nugraha, F.R.; Wibowo, U.A. Porous Architecture Evaluation of Silk Fibroin Scaffold from Direct Dissolution Salt Leaching Method. Symp. 2020, 391, 1900187, doi:10.1002/masy.201900187.
- Khoramgah, M.S.; Ranjbari, J.; Abbaszadeh, H.-A.; Mirakabad, F.S.T.; Hatami, S.; Hosseinzadeh, S.; Ghanbarian, H. Freeze-dried multiscale porous nanofibrous three dimensional scaffolds for bone regenerations. BioImpacts 2020, 10, 73–85, doi:10.34172/bi.2020.10.
- Madrid, A.P.M.; Vrech, S.M.; Sanchez, M.A.; Rodriguez, A.P. Advances in additive manufacturing for bone tissue engineering scaffolds. Sci. Eng. C 2019, 100, 631–644, doi:10.1016/j.msec.2019.03.037.
- Gaspar, V.M.; Lavrador, P.; Borges, J.; Oliveira, M.B.; Mano, J.F. Advanced Bottom‐Up Engineering of Living Architectures. Mater. 2020, 32, e1903975, doi:10.1002/adma.201903975.
- Elbert, D.L. Bottom-up tissue engineering. Opin. Biotechnol. 2011, 22, 674–680, doi:10.1016/j.copbio.2011.04.001.
- Silva, A.S.; Santos, L.F.; Mendes, M.C.; Mano, J.F. Multi-layer pre-vascularized magnetic cell sheets for bone regeneration. Biomaterials 2020, 231, 119664, doi:10.1016/j.biomaterials.2019.119664.
- Chuah, Y.J.; Tan, J.R.; Wu, Y.; Lim, C.S.; Hee, H.T.; Kang, Y.; Wang, D.-A. Scaffold-Free tissue engineering with aligned bone marrow stromal cell sheets to recapitulate the microstructural and biochemical composition of annulus fibrosus. Acta Biomater. 2020, 107, 129–137, doi:10.1016/j.actbio.2020.02.031.
- Ahmad, T.; Byun, H.; Lee, J.; Perikamana, S.K.M.; Shin, Y.M.; Kim, E.M.; Shin, H. Stem cell spheroids incorporating fibers coated with adenosine and polydopamine as a modular building blocks for bone tissue engineering. Biomaterials 2020, 230, 119652, doi:10.1016/j.biomaterials.2019.119652.
- Kronemberger, G.S.; Matsui, R.A.M.; Miranda, G.D.A.S.D.C.E.; Granjeiro, J.M.; Baptista, L.S. Cartilage and bone tissue engineering using adipose stromal/stem cells spheroids as building blocks. World J. Stem Cells 2020, 12, 110–122, doi:10.4252/wjsc.v12.i2.110.
- Toda, S.; Frankel, N.W.; Lim, W.A. Engineering cell–cell communication networks: Programming multicellular behaviors. Opin. Chem. Biol. 2019, 52, 31–38, doi:10.1016/j.cbpa.2019.04.020.
- Amaral, A.J.; Pasparakis, G. Cell membrane engineering with synthetic materials: Applications in cell spheroids, cellular glues and microtissue formation. Acta Biomater. 2019, 90, 21–36, doi:10.1016/j.actbio.2019.04.013.
- Qu, M.; Jiang, X.; Zhou, X.; Wang, C.; Wu, Q.; Ren, L.; Zhu, J.; Zhu, S.; Tebon, P.; Sun, W.; et al. Stimuli‐Responsive Delivery of Growth Factors for Tissue Engineering. Heal. Mater. 2020, 9, e1901714, doi:10.1002/adhm.201901714.
- Tang, J.D.; Mura, C.; Lampe, K.J. Stimuli-Responsive, Pentapeptide, Nanofiber Hydrogel for Tissue Engineering. Am. Chem. Soc. 2019, 141, 4886–4899, doi:10.1021/jacs.8b13363.
- Fathi‐Achachelouei, M.; Keskin, D.; Bat, E.; Vrana, N.E.; Tezcaner, A. Dual growth factor delivery using PLGA nanoparticles in silk fibroin/PEGDMA hydrogels for articular cartilage tissue engineering. Biomed. Mater. Res. Part B: Appl. Biomater. 2019, 108, 2041–2062, doi:10.1002/jbm.b.34544.
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Kim, Y.K.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 013001, doi:10.1088/1758-5090/8/1/013001.
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018, 36, 384–402, doi:10.1016/j.tibtech.2017.10.015.
- Divakar, D.D.; Jastaniyah, N.T.; Altamimi, H.G.; Alnakhli, Y.O.; Muzaheed; Alkheraif, A.A.; Haleem, S. Enhanced antimicrobial activity of naturally derived bioactive molecule chitosan conjugated silver nanoparticle against dental implant pathogens. J. Biol. Macromol. 2018, 108, 790–797, doi:10.1016/j.ijbiomac.2017.10.166.
- Nguyen, M.A.; Camci-Unal, G. Unconventional Tissue Engineering Materials in Disguise. Trends Biotechnol. 2020, 38, 178–190, doi:10.1016/j.tibtech.2019.07.014.
- Levato, R.; Jungst, T.; Scheuring, R.G.; Blunk, T.; Groll, J.; Malda, J. From Shape to Function: The Next Step in Bioprinting. Mater. 2020, 32, e1906423, doi:10.1002/adma.201906423.
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Bone Jt. Surg. 2018, 6, 90–99.
- Raquel Maia, F.; Correlo, V.M.; Oliveira, J.M.; Reis, R.L. Chapter 32—Natural Origin Materials for Bone Tissue Engineering: Properties, Processing, and Performance. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Boston, MA, USA, 2019; pp 535–558.
- Wang, W.; Yeung, K.W. Bone grafts and biomaterials substitutes for bone defect repair: A review. Mater. 2017, 2, 224–247, doi:10.1016/j.bioactmat.2017.05.007.
- Souza, A.T.P.; Lopes, H.B.; Freitas, G.P.; Ferraz, E.P.; Oliveira, F.S.; Almeida, A.L.G.; Weffort, D.; Beloti, M.M.; Rosa, A.L. Role of embryonic origin on osteogenic potential and bone repair capacity of rat calvarial osteoblasts. Bone Miner. Metab. 2020, 38, 481–490, doi:10.1007/s00774-020-01090-5.
- Vasquez-Sancho, F.; Abdollahi, A.; Damjanovic, D.; Catalan, G. Flexoelectricity in Bones. Mater. 2018, 30, 1705316, doi:10.1002/adma.201705316.
- Núñez-Toldrà, R.; Vasquez-Sancho, F.; Barroca, N.; Catalan, G. Investigation of The Cellular Response to Bone Fractures: Evidence for Flexoelectricity. Rep. 2020, 10, 1–10, doi:10.1038/s41598-019-57121-3.
- Lin, C.-C.; Chang, W.H.-S.; Cheng, T.-M.; Chiu, L.-H.; Wang, Y.-H.; Lin, C.-A.J.; Ho, Y.-S.; Zuo, C.S.; Wang, Y.-M.; Lai, W.-F.T. Two new, near-infrared, fluorescent probes as potential tools for imaging bone repair. Rep. 2020, 10, 1–10, doi:10.1038/s41598-020-59522-1.
- Saha, S.; Pramanik, K.; Biswas, A. Silk fibroin coated TiO2 nanotubes for improved osteogenic property of Ti6Al4V bone implants. Sci. Eng. C 2019, 105, 109982, doi:10.1016/j.msec.2019.109982.
- Qin, T.; Li, X.; Long, H.; Bin, S.; Xu, Y. Bioactive Tetracalcium Phosphate Scaffolds Fabricated by Selective Laser Sintering for Bone Regeneration Applications. Materials 2020, 13, 2268, doi:10.3390/ma13102268.
- Zhou, J.; Gao, C.; Feng, P.; Xiao, T.; Shuai, C.; Peng, S. Calcium sulfate bone scaffolds with controllable porous structure by selective laser sintering. Porous Mater. 2015, 22, 1171–1178, doi:10.1007/s10934-015-9993-x.
- Shuai, C.; Gao, C.; Nie, Y.; Hu, H.; Zhou, Y.; Peng, S. Structure and properties of nano-hydroxypatite scaffolds for bone tissue engineering with a selective laser sintering system. Nanotechnology 2011, 22, 285703, doi:10.1088/0957-4484/22/28/285703.
- Duan, B.; Wang, M.; Zhou, W.Y.; Cheung, W.L.; Li, Z.Y.; Lu, W.W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010, 6, 4495–4505, doi:10.1016/j.actbio.2010.06.024.
- Simpson, R.L.; Wiria, F.E.; Amis, A.A.; Chua, C.K.; Leong, K.F.; Hansen, U.N.; Chandrasekaran, M.; Lee, M.W. Development of a 95/5 poly(L-lactide-co-glycolide)/hydroxylapatite and β-tricalcium phosphate scaffold as bone replacement material via selective laser sintering. Biomed. Mater. Res. B 2008, 84B, 17-25.
- Cox, S.C.; Thornby, J.A.; Gibbons, G.J.; Williams, M.A.; Mallick, K.K. 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Sci. Eng. C 2015, 47, 237–247, doi:10.1016/j.msec.2014.11.024.
- Ahn, M.-K.; Moon, Y.-W.; Maeng, W.-Y.; Koh, Y.-H.; Kim, H.-E. Design and Production of Continuously Gradient Macro/Microporous Calcium Phosphate (CaP) Scaffolds Using Ceramic/Camphene-Based 3D Extrusion. Materials 2017, 10, 719, doi:10.3390/ma10070719.
- Chow, L.C.; Takagi, S. A natural bone cement—A laboratory novelty led to the development of revolutionary new biomaterials. Res. Natl. Inst. Stand. Technol. 2001, 106, 1029–1033, doi:10.6028/jres.106.053.
- Kretlow, J.D.; Mikos, A.G. Review: Mineralization of Synthetic Polymer Scaffolds for Bone Tissue Engineering. Tissue Eng. 2007, 13, 927–938, doi:10.1089/ten.2006.0394.
- Rho, J.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med Eng. Phys. 1998, 20, 92–102, doi:10.1016/s1350-4533(98)00007-1.
- Currey, J.; Butler, G. The mechanical properties of bone tissue in children. JBJS 1975, 57, 810–814, doi:10.2106/00004623-197557060-00015.
- Vogel, H.G. Influence of Maturation and Aging on Mechanical and Biochemical Parameters of Rat Bone. Gerontology 1979, 25, 16–23, doi:10.1159/000212316.
- Dunlop, J.W.C.; Fratzl, P. Biological Composites. In Annual Review of Materials Research; Clarke, D.R., Ruhle, M.; Zok, F., Eds.; Annual Reviews: Palo Alto, CA, USA, 2010; Volume 40, pp 1–24.
- Hardy, J.G.; Torres-Rendon, J.G.; Leal-Egaña, A.; Walther, A.; Schlaad, H.; Cölfen, H.; Scheibel, T. Biomineralization of Engineered Spider Silk Protein-Based Composite Materials for Bone Tissue Engineering. Materials 2016, 9, 560, doi:10.3390/ma9070560.
- Lee, J. Y.; Choo, J. E.; Choi, Y. S.; Park, J. B.; Min, D. S.; Lee, S. J.; Rhyu, H. K.; Jo, I. H.; Chung, C. P.; Park, Y. J., Assembly of collagen-binding peptide with collagen as a bioactive scaffold for osteogenesis in vitro and in vivo. Biomaterials 2007, 28 (29), 4257-4267, doi: 1016/j.biomaterials.2007.05.040.
- Dinjaski, N.; Plowright, R.; Zhou, S.; Belton, D.J.; Perry, C.C.; Kaplan, D.L. Osteoinductive recombinant silk fusion proteins for bone regeneration. Acta Biomater. 2017, 49, 127–139, doi:10.1016/j.actbio.2016.12.002.
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915, doi:10.1016/j.biomaterials.2006.01.017.
- Giachelli, C.M.; Steitz, S. Osteopontin: A versatile regulator of inflammation and biomineralization. Matrix Biol. 2000, 19, 615–622, doi:10.1016/s0945-053x(00)00108-6.
- Jin, R.; Wang, Y.; Liu, Y.; Ding, C.; Xie, J.; Li, J. Biomineralization and osteogenic differentiation modulated by substrate stiffness. Polym. J. 2020, 122, 109395, doi:10.1016/j.eurpolymj.2019.109395.
- Midha, S.; Kumar, S.; Sharma, A.; Kaur, K.; Shi, X.; Naruphontjirakul, P.; Jones, J.R.; Ghosh, S. Silk fibroin-bioactive glass based advanced biomaterials: Towards patient-specific bone grafts. Mater. 2018, 13, 055012, doi:10.1088/1748-605x/aad2a9.
- Hench, L.L. The story of Bioglass®. Mater. Sci. Mater. Med. 2006, 17, 967–978.
- Mertgen, A.-S.; Trossmann, V.T.; Guex, A.G.; Maniura-Weber, K.; Scheibel, T.; Rottmar, M. Multifunctional Biomaterials: Combining Material Modification Strategies for Engineering of Cell-Contacting Surfaces. ACS Appl. Mater. Interfaces 2020, 12, 21342–21367, doi:10.1021/acsami.0c01893.
- Le Saux, G.; Magenau, A.; Böcking, T.; Gaus, K.; Gooding, J.J. The Relative Importance of Topography and RGD Ligand Density for Endothelial Cell Adhesion. PLoS ONE 2011, 6, e21869, doi:10.1371/journal.pone.0021869.
- Wohlrab, S.; Müller, S.; Schmidt, A.; Neubauer, S.; Kessler, H.; Leal-Egaña, A.; Scheibel, T. Cell adhesion and proliferation on RGD-modified recombinant spider silk proteins. Biomaterials 2012, 33, 6650–6659, doi:10.1016/j.biomaterials.2012.05.069.
- Bessa, P.C.; Balmayor, E.R.; Azevedo, H.S.; Nürnberger, S.; Casal, M.; Van Griensven, M.; Reis, R.L.; Redl, H. Silk fibroin microparticles as carriers for delivery of human recombinant BMPs. Physical characterization and drug release. Tissue Eng. Regen. Med. 2010, 4, 349–355, doi:10.1002/term.245.
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from laboratory to clinic, part II (BMP delivery). Tissue Eng. Regen. Med. 2008, 2, 81–96, doi:10.1002/term.74.
- Gomes, S.; Gallego-Llamas, J.; Leonor, I.B.; Mano, J.F.; Reis, R.L.; Kaplan, D.L. In Vivo Biological Responses to Silk Proteins Functionalized with Bone Sialoprotein. Biosci. 2013, 13, 444–454, doi:10.1002/mabi.201200372.
- Neubauer, V.J.; Scheibel, T. Spider Silk Fusion Proteins for Controlled Collagen Binding and Biomineralization. ACS Biomater. Sci. Eng. 2020, 6, 5599–5608, doi:10.1021/acsbiomaterials.0c00818.
- Yousaf, S.S.; Houacine, C.; Khan, I.; Ahmed, W.; Jackson, M.J. Chapter 11—Importance of biomaterials in biomedical engineering. In Advances in Medical and Surgical Engineering; Ahmed, W., Phoenix, D.A., Jackson, M.J., Charalambous, C.P., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp 149–176.
This entry is adapted from the peer-reviewed paper 10.3390/ma14030674