Biodiesel produced through catalytic transesterification of triglycerides from edible and non-edible oils and alcohol is considered a clean and renewable alternative to traditional petro-diesel. Homogeneous alkaline catalysts have been widely used in this reaction due to their high activity. Optimization studies have been carried out to improve the biodiesel yields by modulation of the reaction conditions such as temperature, alcohol to oil ratio, catalysts concentration, time, and agitation speed. Several designs of experiments can be used to model to achieve this.
- biodiesel
- alkaline-earth metals
- heterogeneous catalysts
- transesterification
- basicity
- operation conditions
- recycle
- vegetable oils
1. Introduction
Nowadays, oil is the primary energy source worldwide since it is used mainly as fuel for transportation and electric power plants. The world population’s growth has caused an increase in demand for petroleum-derived products, a non-renewable source. The mathematical models have projected that oil could be scarce between 2048 and 2058 [1]. On the one hand, economic dependence on oil has produced war, political, and economic conflicts in oil-producing countries. Particularly, in 2020, the oil prices have been highly unstable due to the pandemic caused by the novel virus SARS-CoV-2 and the excessive oil production, affecting the economies of the producer countries [2]. Additionally, the burning of fossil fuels is responsible for the emission of greenhouse gases which are the cause of the climate crisis. In 2018, approximately 23–29% of the total CO2 generated, came from fuels used in transportation and has reached more than 400 ppm in the atmosphere, which has increased the average terrestrial temperature [3][4][5][6][7]. In this sense, humans must mitigate and delay negative effects through the education, green chemistry, and development of sustainable and renewable technologies and actions. Following the objectives of the Paris Agreements in 2016, an annual increase in the development and use of renewable energy sources has been promoted thanks to the support and subsidies in biofuel prices by governments [8][9][10]. Therefore, the use of biomass as an alternative, renewable and clean source has drawn the attention of scientists to reduce environmental impact. Particularly the development of biofuels seems to be a viable, economical, clean, and potentially applicable option to rural societies [11][12].
1.1. Biofuels
Biofuels are generated from different sources of biomass, such as crops, seeds, agroindustry wastes, and algae, which have the characteristic of being renewable, not toxic, accessible, cheap, with lower sulfur and nitrogen content, and a faster reintegration of CO2 emitted to the carbon cycle [11][13][14][15]. However, the use of biofuels could not cover the entire demand for fuels on the planet because their production depends on the quality, availability, and production of biomass. In this way, biofuels can potentially be used in small towns or mixed with fossil fuels not only to reduce the consumption and import of the latter but also for rural economic activation purposes [16][17]. The transformation of biomass in biorefineries can lead to value-added products that are used as additives or raw material for other processes and products [18].
Biofuels are divided into four generations (1G, 2G, 3G, and 4G) depending on the source from which they were synthesized, and all seek to reduce the oxygen content of the molecules that make up the biomass to increase energy density and increase the molecular weight of hydrocarbons [19]. 1G biofuels are obtained from edible or inedible crops, 2G biofuels from biomass waste such as sawdust or wood. 3G and 4G biofuels are produced from algae and microorganisms that can be genetically modified to improve their characteristics [20][21]. The biodiesel obtained through the transesterification of vegetable oils (1G) is the most produced biofuel worldwide. Consequently, several studies have focused on developing the optimization of this process and its catalysts [22][23][24]. The latter is an important factor in the improvement of biodiesel production systems since they determine the quality and quantity of biodiesel, as well as the ecological impact that the plant can have. In this sense, the main objective of this work is to review and analyze the synergy of operating conditions and the development of catalysts that optimize the production of biofuels through transesterification reactions of vegetable oils.
1G biofuels, also called conventional biofuels, are produced from agronomic crops from which sugars and starches are extracted to generate alcohols (bioethanol) and vegetable oils that are transformed into biodiesel. Biofuels are safe, have good quality due to the high cetane or octane numbers, and can be mixed with fossil fuel loads to decrease the carbon footprint [25]. These biofuels are the most developed and used industrially due to their reduced production costs and relative simplicity in their processes and have a carbon footprint near to neutral [8][17][26]. Biodiesel is generated from edible and non-edible vegetable oils such as canola, corn, soybean, castor, jatropha, olive, coconut, peanut, palm, cotton, cane, wheat, and fruit oils, and even from oil generated from algae and microalgae [27]. Also, it can be generated from animal fat and edible waste oils from restaurants or businesses. However, it is well known that obtaining biodiesel from edible oils creates competition with the food industry, so that food prices and their availability could be compromised by fuel demand. Likewise, they present environmental impacts that lead to deforestation for cultivation and massive use of fertilizers [28][29]. Hence, the use of wasted oils and non-edible oils would help to reduce competition with food production and increase sustainable production in developing countries [30][31][32].
Biodiesel is mainly composed of a combination of fatty acid methyl esters (FAME) or fatty acid ethyl esters (FAEE) derived from triglycerides and fatty acids that make up vegetable oils and animal fats that can be incorporated into petroleum diesel fillers [33]. This fuel has the advantage of being biodegradable, renewable, presenting low toxicity, high energy value (39–41 MJ/kg), and having more lubrication than conventional petroleum diesel. However, the molecular structures of fatty acid chains, such as length or unsaturation, affect the quality of biodiesel. At low temperatures, it can become a gel that could cover the filters, or the unsaturation of the chains can oxidize and degrade the fuel [34][35]. Therefore, to improve the quality of biodiesel it is necessary to conduct studies on the process conditions, kinetics, and functionalities of the catalysts.
1.2. Transesterification of Triglycerides
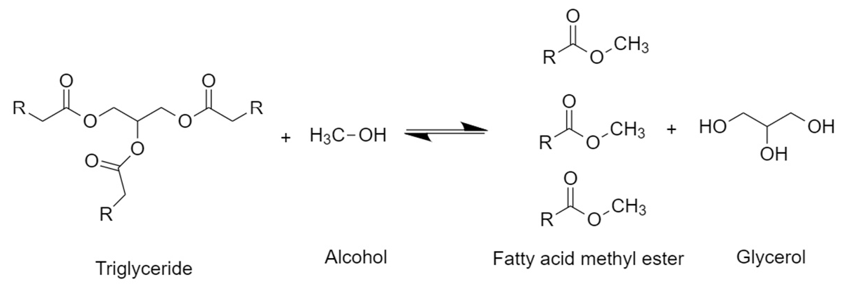
The transesterification of triglycerides from vegetable oils with alcohols in the presence of a catalyst to generate FAME and glycerol as a by-product is shown in Figure 1. Methanol and ethanol are the most used alcohols for these reactions since they can be formed from fermentation, so they are accessible and present a low cost; besides, they easily dissolve homogeneous catalysts [36]. Because the reaction is reversible, an excess of alcohol is necessary to shift the equilibria towards the products [37].
Figure 1. General scheme of the transesterification reaction of triglycerides with methanol.
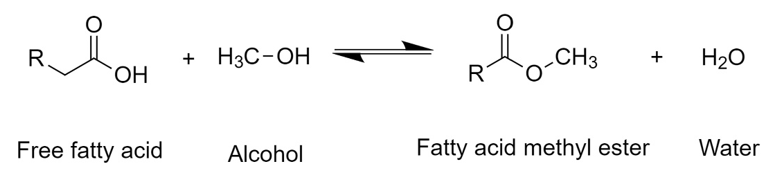
First, a triglyceride reacts with alcohol to give a molecule of fatty acid methyl ester and one of diglyceride. The latter reacts with another molecule of alcohol to generate another molecule of fatty acid methyl ester and a monoglyceride, which repeats the same procedure. Therefore, stoichiometrically requires a 3:1 molar ratio of alcohol to triglyceride to form three molecules of FAME and one of glycerol [38][39]. Commonly, transesterification reactions at the industrial level are carried out by a homogeneous catalyst with a strong basic character, such as NaOH and KOH. However, strong acids such as H2SO4 and HCl, enzymes like lipases, and heterogeneous catalysts can be used as well [40][41][42]. After the reaction, two phases are generated: the biodiesel (FAME) phase at the top and the glycerol phase at the bottom. This latter consists of a mixture of compounds present from the vegetable oil such as water, organic compounds which gave color and flavor to the oil, inorganic salts, alcohol, glycerides, and catalyst. Hence, after simple decantation, this by-product can be separated and then purified to be used as raw material for other processes. Free fatty acids (FFA) present in the oil can be transformed into esters by an esterification reaction in the presence of an acid catalyst such as H2SO4 (Figure 2).
Figure 2. General scheme of the esterification reaction of free fatty acids with methanol.
In this reaction, the oxygen of the carbonyl group is first protonated by the acid catalyst generating a cationic complex. The oxygen in the alcohol then nucleophilically attacks the carbon in the carbonyl group of the complex. Finally, hydrogen bonds to the hydroxyl group of the acid, forming water and a FAME molecule [43][44]. In both processes, it has been seen that the reaction performance depends mainly on the molar ratio between alcohol and triglyceride (or FFA), temperature, and the amount of catalyst used [37][45].
1.3. Catalysts
The use of homogenous catalysts has advantages such as high activity and availability, low cost, moderate operating conditions (atmospheric pressure and 50–70 °C), and production of high-quality biodiesel. On the one hand, glycerol forms a thick organic phase that precipitates, so it is relatively easy to separate from biodiesel by centrifugation or decantation. On the other hand, the methanol that is diluted with biodiesel can be recovered by distillation and reused. However, since the homogeneous catalyst is in the same phase as the oil, FAME, and glycerol; it must be separated by a purification process [46]. To achieve this, large amounts of water are required, which results in contamination, increase in the cost of the process, and there is a risk that it will result in the saponification of FFA consuming the catalyst and making the separation of biodiesel difficult [47][48]. Thus, it has been determined that vegetable oil must have a maximum of 1 wt.% FFA and less than 0.06 wt.% water to maximize FAME formation [49]. In parallel, strong acids can also be used as homogeneous catalysts, preferably in loads of used oil or animal fats, since they have a high FFA content [50]. Like the basic catalysts, their operating conditions are moderate and require high alcohol/oil molar ratios to obtain acceptable yields [51]. Nevertheless, unlike basic catalysts, acid catalysts do not run the risk of unwanted saponification reactions and can perform transesterification and esterification simultaneously [52][53][54]. The acid catalysts can directly produce biodiesel from low-grade, highly acidic, and water-containing oils. Despite this, their activity is relatively low in transesterification compared to basic catalysts. Moreover, they are highly corrosive, toxic and the separation processes involved are more complex and require greater amounts of water than basic catalysts. Water addition provokes a rapid separation of the two phases by the formation of hydrogen bonds between solvent and water that are energetically more favorable than the van der Waals interactions between alcohol and biodiesel. Hence it interrupts the emulsifying action of alcohol with an immediate separation of glycerol from biodiesel [53]. Optimization studies in biofuel production have used a different design of experiment methods [55]. In current literature, it is possible to find several studies that have compared and used different types of oils and methods to find the relationships between operational conditions and optimize their values. For example, Veljkovic et al. [56] conducted a study in which the methods of Box-Behnken, face-centered composite, and full factorial in the transesterification of sunflower oil with ethanol in the presence of NaOH were compared. The reaction temperature, the amount of NaOH, and the ethanol/oil molar ratio were modified to find the optimal conditions. This study determined that if the ethanol/oil molar ratio is 12:1 and the amount of NaOH is 1.25 wt.%, the reaction would reach a 98% yield in temperature ranges from 25 to 75 °C. They also concluded that the Box-Behnken and face-centered composite methods are recommended to reduce the number of experiments and predict reliable results in the optimization of the biodiesel production process. Some examples that used other experimental designs are exposed in Table 1.
Table 1. Optimization of biofuel production with different designs of experiments.
|
Oil System |
Design of Experiment |
Optimal |
Yield (%) |
Catalysts |
Reference |
|
Sunflower Oil |
Box-Behnken, Face Centered Central Full Factorial |
Temperature: 75 °C Ethanol/Oil: 12:1 Catalyst loading: 1.25 wt.% |
97.6 |
NaOH |
[56] |
|
Castor Oil |
Taguchi |
Time: 60 min Temperature: 50 °C Catalyst loading: 1 wt.% Methanol/oil: 20:1 Agitation speed: 700 rpm |
90.83 |
H2SO4 |
[57] |
|
Mahogany Seed Oil |
Taguchi |
Methanol/oil: 9:1 Catalyst loading: 0.5 wt.% Temperature: 60 °C Agitation speed: 300 rpm |
96.8 |
NaOH |
[58] |
|
Ceiba Pentandra Oil |
Box-Behnken |
Methanol/oil: 60:1 Catalyst loading: 0.84 wt.% Time: 6.46 min Agitation speed: 800 rpm Microwaved to 100 °C |
95.42 |
KOH |
[59] |
|
Sardine Fish Oil |
Box-Behnken |
Temperature: 150 °C Methanol/oil: 6:1 Catalyst loading: 1.25 wt.% Time: 25 min |
98.1 |
KOH |
[60] |
|
Karanja Oil |
Box-Behnken |
Methanol/oil: 10.44:1 Catalyst loading: 1.22 wt.% Time: 90.78 min Temperature: 66.8 °C |
91.05 |
KOH |
[61] |
|
Mixture of Pongamia and Neem Oils |
Central Composite |
Time: 77 min Catalyst loading: 0.67 wt. % Methanol/oil: 6:1 |
86.3 |
NaOH |
[62] |
To avoid or minimize the inconveniences of the conventional homogenous catalysts, the use of heterogeneous catalysts has been proposed, which have shown potential for these reactions. Likewise, it has been considered that these catalysts could be used soon from small agricultural plants to be incorporated into refineries to mix the biodiesel with the conventional diesel loads. The development of heterogeneous catalysts has garnered attention because they are easy to separate by physical methods, low water consumption, they can be used in continuous reactors and reused after being recovered, they are not corrosive, present less sensitive to the presence of water, and are non-hazardous to the environment [51][63][64]. However, their catalytic activities remain low compared to homogeneous catalysts due to the amount and strength of active sites and the problems of matter transfer in the three-phase system (catalyst-oil-alcohol). Besides, there may be leaching of active sites or deactivation of sites by CO2 from the air [65]. Consequently, this has motivated different investigations to obtain sufficiently active catalysts that can be recovered as well as the reaction conditions and types of reactors.
Heterogeneous catalysts can have basic or acidic functionalities. The basic solids include a wide variety of catalysts such as alkaline earth, which can be doped with alkali metals, mixed or supported over Al2O3, SiO2, and hydrotalcites [66][67][68]. Additionally, transition metal oxides, zeolites, or activated carbon can also function as catalysts with acidic functionalities [69]. The use of solid catalysts derived from biomass such as diatomic earth, animal bones, seashells, or eggshell, has been proposed as more ecological alternatives [70]. This vast catalog of catalysts allows choosing the best system depending on the nature and quality of the oil, its availability, and the economic situation of the biorefinery or rural plant. As alkaline earth metal oxides have presented relative advantages over the other materials, they can be potentially used due to their availability, price, and catalytic activity.
This entry is adapted from the peer-reviewed paper 10.3390/en14041031
References
- Al Rashdan, M.; Al Zubi, M.; Al Okour, M. Effect of Using New Technology Vehicles on the World’s Environment and Petroleum Resources. Ecol. Eng. 2019, 20, 16–24, doi:10.12911/22998993/93945.
- Oil Prices. Available online: www.oilprice.com (accessed on 19 April 2020).
- Tans, P.; Keeling, R. Trends in atmospheric carbon dioxide. Available online: http://www.esrl.noaa.gov/gmd/ccgg/ trends/mlo.html#mlo_full (accessed on 7 January 2012).
- Ballesteros, M.; Manzanares, P. Liquid Biofuels. In The Role of Bioenergy in the Bioeconomy; Elago, C., Caldés, N., Lechón, Y., Eds.; Academic Press: Amsterdam, The Netherlands, 2019; pp. 113–144.
- Oh, Y.-K.; Hwang, K.-R.; Kim, C.; Kim, J.R.; Lee, J.-S. Recent developments and key barriers to advanced biofuels: A short review. Technol. 2018, 257, 320–333, doi:10.1016/j.biortech.2018.02.089.
- Global Energy & CO2 Status Report. Available online: https://www.iea.org/geco/emissions/ (accessed on 19 April 2020).
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809, doi:10.1016/j.enpol.2012.10.046.
- Manaf, I.S.A.; Embong, N.H.; Khazaai, S.N.M.; Rahim, M.H.A.; Yusoff, M.M.; Lee, K.T.; Maniam, G.P. A review for key challenges of the development of biodiesel industry. Energy Convers. Manag. 2019, 185, 508–517, doi:10.1016/j.enconman.2019.02.019.
- Paris Agreement, U.N. The Paris Agreement. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 19 April 2019).
- Cespi, D.; Esposito, I.; Cucciniello, R.; Anastas, P.T. Beyond the beaker: Benign by design society. Res. Green Sustain. Chem. 2020, 3, 100028, doi:10.1016/j.crgsc.2020.100028.
- Sunde, K.; Brekke, A.; Solberg, B. Environmental impacts and costs of woody Biomass-to-Liquid (BTL) production and use—A review. Policy Econ. 2011, 13, 591–602, doi:10.1016/j.forpol.2011.05.008.
- Chanthawong, A.; Dhakal, S. Liquid Biofuels Development in Southeast Asian Countries: An Analysis of Market, Policies and Challenges. Waste Biomass- Valorization 2015, 7, 157–173, doi:10.1007/s12649-015-9433-9.
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Kay, A.; Mills-Lamptey, B. Effect of Solvent Extraction Parameters on the Recovery of Oil From Spent Coffee Grounds for Biofuel Production. Waste Biomass- Valorization 2019, 10, 253–264, doi:10.1007/s12649-017-0061-4.
- Correa, D.F.; Beyer, H.L.; Possingham, H.P.; Thomas-Hall, S.R.; Schenk, P.M. Biodiversity impacts of bioenergy production: Microalgae vs. first generation biofuels. Sustain. Energy Rev. 2017, 74, 1131–1146, doi:10.1016/j.rser.2017.02.068.
- Alalwan, H.A.; Alminshid, A.H.; Aljaafari, H.A. Promising evolution of biofuel generations. Subject review. Energy Focus 2019, 28, 127–139, doi:10.1016/j.ref.2018.12.006.
- Hill, J.; Nelson, E.; Tilman, D.; Polasky, S.; Tiffany, D.G. Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc. Natl. Acad. Sci. 2006, 103, 11206–11210, doi:10.1073/pnas.0604600103.
- Van Gerpen, J. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107, doi:10.1016/j.fuproc.2004.11.005.
- Parada, M.P.; Osseweijer, P.; Duque, J.A.P. Sustainable biorefineries, an analysis of practices for incorporating sustainability in biorefinery design. Crop. Prod. 2017, 106, 105–123, doi:10.1016/j.indcrop.2016.08.052.
- Abdullah, B.; Muhammad, S.A.F.S.; Shokravi, Z.; Ismail, S.; Kassim, K.A.; Mahmood, A.N.; Aziz, M.A. Fourth generation biofuel: A review on risks and mitigation strategies. Sustain. Energy Rev. 2019, 107, 37–50, doi:10.1016/j.rser.2019.02.018.
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.-H. State of the art and prospective of lipase-catalyzed transesterification reaction for biodiesel production. Energy Convers. Manag. 2017, 141, 339–353, doi:10.1016/j.enconman.2016.09.049.
- Ngoie, W.I.; Oyekola, O.O.; Ikhu-Omoregbe, D.; Welz, P.J. Valorisation of Edible Oil Wastewater Sludge: Bioethanol and Biodiesel Production. Waste Biomass- Valorization 2019, 11, 2431–2440, doi:10.1007/s12649-019-00633-w.
- Tajuddin, N.; Lee, A.; Wilson, K. Production of biodiesel via catalytic upgrading and refining of sustainable oleagineous feedstocks. In Handbook of Biodiesel Production, 2nd ed.; Luque, R.,Lin, C.S.K.; Wilson, K.; Clark, J.; Woodhead Publishing Series in Energy: Cambridge, England, 2016; pp. 121–164.
- García, I. Feedstocks and challenges to biofuel development; In Handbook of Biodiesel Production, 2nd ed.; Luque, R.,Lin, C.S.K.; Wilson, K.; Clark, J.; Woodhead Publishing Series in Energy: Cambridge, England, 2016; pp. 85–118.
- Le Anh, T.; Reksowardojo, I.; Wattanavichien, K. Utilization of biofuels in diesel engines; In Handbook of Biodiesel Production, 1st ed.; Luque, R., Campelo, J.; Clark, J.; Woodhead Publishing: Cambridge, England, 2011; pp. 611-646.
- Marchetti, J.; Miguel, V.; Errazu, A. Possible methods for biodiesel production. Sustain. Energy Rev. 2007, 11, 1300–1311, doi:10.1016/j.rser.2005.08.006.
- Bateni, H.; Bateni, F.; Karimi, K. Effects of Oil Extraction on Ethanol and Biogas Production from Eruca sativa Seed Cake. Waste Biomass- Valorization 2016, 8, 1897–1905, doi:10.1007/s12649-016-9731-x.
- Demirbas, M.F. Biofuels from algae for sustainable development. Energy 2011, 88, 3473–3480, doi:10.1016/j.apenergy.2011.01.059.
- Ajanovic, A. Biofuels versus food production: Does biofuels production increase food prices? Energy 2011, 36, 2070–2076, doi:10.1016/j.energy.2010.05.019.
- Garlapati, V.K.; Tewari, S.; Ganguly, R. Life Cycle Assessment of First-, Second-Generation, and Microalgae Biofuels. In Advances in Feedstock Conversion Technologies for Alternative Fuels and Bioproducts; 1st ; Hosseini, M.; Woodhead Publishing Series in Energy: Cambridge, England, 2019; pp. 355–371.
- Demirbas, A. Biodiesel production from vegetable oils via catalytic and non-catalytic supercritical methanol transesterification methods. Energy Combust. Sci. 2005, 31, 466–487, doi:10.1016/j.pecs.2005.09.001.
- Borges, M.; Díaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Sustain. Energy Rev. 2012, 16, 2839–2849, doi:10.1016/j.rser.2012.01.071.
- Khan, T.M.Y.; Atabani, A.E.; Irfananjumbadruddin, I.; Badarudin, A.; Khayoon, M.S.; Triwahyono, S. Recent scenario and technologies to utilize non-edible oils for biodiesel production. Sustain. Energy Rev. 2014, 37, 840–851, doi:10.1016/j.rser.2014.05.064.
- Joshi, G.; Pandey, J.K.; Rana, S.; Rawat, D.S. Challenges and opportunities for the application of biofuel. Sustain. Energy Rev. 2017, 79, 850–866, doi:10.1016/j.rser.2017.05.185.
- Atabani, A.; Silitonga, A.; Badruddin, I.A.; Mahlia, T.; Masjuki, H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Sustain. Energy Rev. 2012, 16, 2070–2093, doi:10.1016/j.rser.2012.01.003.
- Marwaha, A.; Dhir, A.; Mahla, S.K.; Mohapatra, S.K. An overview of solid base heterogeneous catalysts for biodiesel production. Rev. 2018, 60, 594–628, doi:10.1080/01614940.2018.1494782.
- Tariq, M.; Ali, S.; Khalid, N. Activity of homogeneous and heterogeneous catalysts, spectroscopic and chromatographic characterization of biodiesel: A review. Sustain. Energy Rev. 2012, 16, 6303–6316, doi:10.1016/j.rser.2012.07.005.
- Demirbas, A. Production of biodiesel fuels from linseed oil using methanol and ethanol in non-catalytic SCF conditions. Biomass- Bioenergy 2009, 33, 113–118, doi:10.1016/j.biombioe.2008.04.018.
- Darnoko, D.; Cheryan, M. Kinetics of palm oil transesterification in a batch reactor. Am. Oil Chem. Soc. 2000, 77, 1263–1267, doi:10.1007/s11746-000-0198-y.
- Portha, J.-F.; Allain, F.H.T.; Coupard, V.; Dandeu, A.; Girot, E.; Schaer, E.; Falk, L. Simulation and kinetic study of transesterification of triolein to biodiesel using modular reactors. Eng. J. 2012, 207–208, 285–298, doi:10.1016/j.cej.2012.06.106.
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar based solid acid catalyst for biodiesel production. Catal. A: Gen. 2010, 382, 197–204, doi:10.1016/j.apcata.2010.04.051.
- Di Serio, M.; Tesser, R.; Pengmei, L.; Santacesaria, E. Heterogeneous Catalysts for Biodiesel Production. Energy Fuels 2008, 22, 207–217, doi:10.1021/ef700250g.
- Konwar, L.J.; Boro, J.; Deka, D. Review on latest developments in biodiesel production using carbon-based catalysts. Sustain. Energy Rev. 2014, 29, 546–564, doi:10.1016/j.rser.2013.09.003.
- Kulkarni, M.G.; Gopinath, R.; Meher, L.C.; Dalai, A.K. Solid acid catalyzed biodiesel production by simultaneous esterification and transesterification. Green Chem. 2006, 8, 1056–1062, doi:10.1039/b605713f.
- Gan, S.; Ng, H.K.; Ooi, C.W.; Motala, N.O.; Ismail, M.A.F. Ferric sulphate catalysed esterification of free fatty acids in waste cooking oil. Technol. 2010, 101, 7338–7343, doi:10.1016/j.biortech.2010.04.028.
- Meher, L.; Sagar, D.V.; Naik, S. Technical aspects of biodiesel production by transesterification—a review. Sustain. Energy Rev. 2006, 10, 248–268, doi:10.1016/j.rser.2004.09.002.
- Demirbaş, A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: A survey. Energy Convers. Manag. 2003, 44, 2093–2109, doi:10.1016/s0196-8904(02)00234-0.
- Zabeti, M.; Daud, W.M.A.W.; Aroua, M.K. Activity of solid catalysts for biodiesel production: A review. Fuel Process. Technol. 2009, 90, 770–777, doi:10.1016/j.fuproc.2009.03.010.
- Furuta, S.; Matsuhashi, H.; Arata, K. Biodiesel fuel production with solid superacid catalysis in fixed bed reactor under atmospheric pressure. Commun. 2004, 5, 721–723, doi:10.1016/j.catcom.2004.09.001.
- Van Kasteren, J.H.; Nisworo, A. A process model to estimate the cost of industrial scale biodiesel production from waste cooking oil by supercritical transesterification. Conserv. Recycl. 2007, 50, 442–458, doi:10.1016/j.resconrec.2006.07.005.
- Leung, D.Y.; Wu, X.; Leung, M. A review on biodiesel production using catalyzed transesterification. Energy 2010, 87, 1083–1095, doi:10.1016/j.apenergy.2009.10.006.
- Helwani, Z.; Othman, M.; Aziz, N.; Kim, J.; Fernando, W. Solid heterogeneous catalysts for transesterification of triglycerides with methanol: A review. Catal. A: Gen. 2009, 363, 1–10, doi:10.1016/j.apcata.2009.05.021.
- Ezebor, F.; Khairuddean, M.; Abdullah, A.Z.; Boey, P.L. Oil palm trunk and sugarcane bagasse derived solid acid catalysts for rapid esterification of fatty acids and moisture-assisted transesterification of oils under pseudo-infinite methanol. Technol. 2014, 157, 254–262, doi:10.1016/j.biortech.2014.01.110.
- Takagaki, A.; Toda, M.; Okamura, M.; Kondo, J.N.; Hayashi, S.; Domen, K.; Hara, M. Esterification of higher fatty acids by a novel strong solid acid. Today 2006, 116, 157–161, doi:10.1016/j.cattod.2006.01.037.
- Su, F.; Guo, Y. Advancements in solid acid catalysts for biodiesel production. Green Chem. 2014, 16, 2934–2957, doi:10.1039/c3gc42333f.
- Tavizón-Pozos, J.A.; Ibarra, I.S.; Guevara-Lara, A.; Galán-Vidal, C.A. Application of Design of Experiments in Biofuel Production: A Review. In Design of Experiments for Chemical, Pharmaceutical, Food, and Industrial Applications; IGI Global: Pennsylvania, United States, 2020; pp 77–103.
- Veljković, V.B.; Veličković, A.V.; Avramović, J.M.; Stamenković, O.S. Modeling of biodiesel production: Performance comparison of Box–Behnken, face central composite and full factorial design. J. Chem. Eng. 2019, 27, 1690–1698, doi:10.1016/j.cjche.2018.08.002.
- Karmakar, B.; Dhawane, S.H.; Halder, G. Optimization of biodiesel production from castor oil by Taguchi design. Environ. Chem. Eng. 2018, 6, 2684–2695, doi:10.1016/j.jece.2018.04.019.
- Dodo, R.; Ause, T.; Dauda, E.; Shehu, U.; Popoola, A. Multi-response optimization of transesterification parameters of mahogany seed oil using grey relational analysis in Taguchi method for quenching application. Heliyon 2019, 5, e02167, doi:10.1016/j.heliyon.2019.e02167.
- Silitonga, A.; Shamsuddin, A.; Mahlia, T.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.; Masjuki, H.; Ong, H.C. Biodiesel synthesis from Ceiba pentandra oil by microwave irradiation-assisted transesterification: ELM modeling and optimization. Energy 2020, 146, 1278–1291, doi:10.1016/j.renene.2019.07.065.
- Kumar, S.A.; Sakthinathan, G.; Vignesh, R.; Banu, J.R.; Al-Muhtaseb, A.H. Optimized transesterification reaction for efficient biodiesel production using Indian oil sardine fish as feedstock. Fuel 2019, 253, 921–929, doi:10.1016/j.fuel.2019.04.172.
- Verma, P.; Sharma, M. Comparative analysis of effect of methanol and ethanol on Karanja biodiesel production and its optimisation. Fuel 2016, 180, 164–174, doi:10.1016/j.fuel.2016.04.035.
- Vinayaka, A.S.; Mahanty, B.; Rene, E.R.; Behera, S.K. Biodiesel production by transesterification of a mixture of pongamia and neem oils. Biofuels 2018, 1–9, doi:10.1080/17597269.2018.1464874.
- Endut, A.; Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Hamid, S.H.A.; Lananan, F.; Kamarudin, M.K.A.; Umar, R.; Juahir, H.; Khatoon, H. Optimization of biodiesel production by solid acid catalyst derived from coconut shell via response surface methodology. Biodeterior. Biodegradation 2017, 124, 250–257, doi:10.1016/j.ibiod.2017.06.008.
- Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Azid, A.; Umar, R.; Juahir, H.; Khatoon, H.; Endut, A. A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production. Sustain. Energy Rev. 2017, 70, 1040–1051, doi:10.1016/j.rser.2016.12.008.
- Kouzu, M.; Hidaka, J.-S. Transesterification of vegetable oil into biodiesel catalyzed by CaO: A review. Fuel 2012, 93, 1–12, doi:10.1016/j.fuel.2011.09.015.
- Dossin, T.F.; Reyniers, M.-F.; Berger, R.J.; Marin, G.B. Simulation of heterogeneously MgO-catalyzed transesterification for fine-chemical and biodiesel industrial production. Catal. B: Environ. 2006, 67, 136–148, doi:10.1016/j.apcatb.2006.04.008.
- Li, H.; Niu, S.; Lu, C.; Li, J. Calcium oxide functionalized with strontium as heterogeneous transesterification catalyst for biodiesel production. Fuel 2016, 176, 63–71, doi:10.1016/j.fuel.2016.02.067.
- D’Cruz, A.; Kulkarni, M.G.; Meher, L.C.; Dalai, A.K. Synthesis of Biodiesel from Canola Oil Using Heterogeneous Base Catalyst. Am. Oil Chem. Soc. 2007, 84, 937–943, doi:10.1007/s11746-007-1121-x.
- Chouhan, A.S.; Sarma, A. Modern heterogeneous catalysts for biodiesel production: A comprehensive review. Sustain. Energy Rev. 2011, 15, 4378–4399, doi:10.1016/j.rser.2011.07.112.
- Ling, J.S.J.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Saptoro, A.; Nolasco-Hipolito, C. A review of heterogeneous calcium oxide based catalyst from waste for biodiesel synthesis. SN Appl. Sci. 2019, 1, 810, doi:10.1007/s42452-019-0843-3.