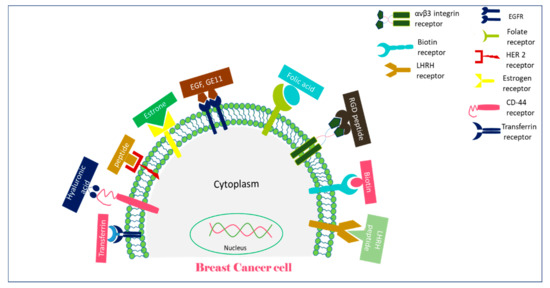
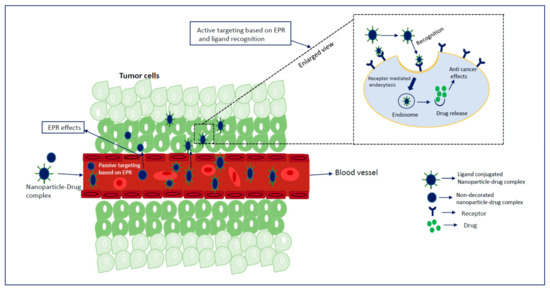
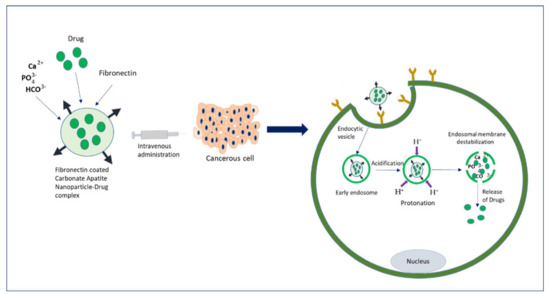
Extensive research on cancer therapeutics introduce several new paradigms to surmount conventional treatment drawbacks over the years. Upon systemic administration, chemotherapeutic drugs have to face several hurdles like premature degradation before reaching the target site, uneven biodistribution, and failure to discriminate healthy and cancer cells in addition to the requirement for their larger and repeated administration [
20,
21,
22,
23]. These barriers are considered responsible for severe unwanted side effects and high treatment costs of conventional chemotherapy [
24,
25]. Thus, a new way of delivering drugs specifically into a target tumor cell is a prerequisite to maximizing therapeutic efficacy and patient compliance. Emerging as a leading contender in the field of targeted drug delivery, NP-based therapeutics comprise therapeutic molecules such as small molecule drugs, peptides/proteins, and nucleic acids (genes and siRNAs) encapsulated into or associated with nanomaterials like liposomes [
26,
27], polymeric NPs [
28,
29], and inorganic NPs [
30,
31]. Since August 2014, 74 completed clinical trials and 57 trails with the status of “active” or “recruited” have been listed in clinicaltrials.gov, over 40,000 citations have been recorded in PubMed on NP-based drug delivery [
21,
32,
33,
34,
35], and many more are on several stages of drug pipelines [
17,
36,
37,
38]. Preclinical studies showed NPs with loaded genes or drug-enhanced antitumor effects with an improved pharmacokinetic profile compared to free drugs [
39,
40,
41] by virtue of the unique physicochemical properties of NPs, including loading capacity for both hydrophobic and hydrophilic drugs, ease of functionalization with targeting moieties and hydrophilic molecules, stability and extended half-lives in the systemic circulation, facilitated tumor accumulation due to their small particle size and EPR effect, enhanced cellular internalization via endocytosis, and quick and sustained release of the drugs in response to various intracellular (e.g., acidic pH) and extracellular (e.g., magnetic field, laser) stimuli [
42,
43,
44]. Additionally, the size, conformation, and surface properties of NPs navigate the trajectory dynamics of the NP-loaded drug complex and are considered fundamental driving forces for improved delivery efficiency [
45,
46,
47]. The passive targeting of NPs capitalizes on the anatomical variances of cancerous tissues. In solid tumors, rapid and abnormal angiogenesis results in an anomalous tumor microenvironment characterized by hyperpermeable tumor vasculature, poor lymphatic drainage, and unorganized extracellular matrix (ECM), allowing the nanocarriers to specifically penetrate through the fenestrate blood vessels and to be retained in the tumor tissue through the EPR effect [
48,
49,
50]. The physicochemical properties and plasma half-life of NPs are considered the main factor for effective passive targeting, taking advantage of the EPR effect. A study showed that NPs with size range of 10–200 nm are enough to avoid clearance by the kidney and reticuloendothelial system (RES) and to facilitate maximum extravasation in the tumor site, whereas NPs with a surface charge slightly negative or neutral are considered ideal for avoiding protein corona effects [
51,
52,
53,
54,
55]. However, passive targeting regulates tumor accumulation without triggering cellular entry, and a recent meta-study showed less than 1% of injected NPs accumulated in the targeted tumor site even with a high EPR effect in the xenografted tumor [
56]. Besides, the idea of the EPR effect does not apply to all tumors. On the other hand, active targeting utilizes homing devices like ligands on the surface of NPs to let them target specifically for binding with specific receptors explicitly expressed on cancerous cells (). The combined effects of EPR and active targeting might increase the retention of drug-loaded NPs at the target tumor site and thus promote cellular drug uptake through receptor-mediated endocytosis (). In the last 10 years, more than 40,000 studies on active targeting of NPs have been reported, with few of them in the clinical trial [
17,
37,
38]. A large number of targeting ligands including proteins, polysaccharide, nucleic acid, peptides, antibodies, and endogenous hormone have been employed for the construction of actively targeting NPs [
57,
58,
59,
60]. Usually, NPs can be functionalized with the targeting moiety by either chemical conjugation or a physical adsorption process. Modification of NPs with specific ligands targeting unique and overexpressed receptors or antigens on tumor cells is supposed to deliver the drug to the target tumor cells without hampering normal cells. Few studies reported that dual receptor-targeting strategies, multivalent binding, and use of a higher density of ligands are beneficial for maximizing binding affinity [
61,
62]. Despite having substantial successful preclinical studies, active targeting suffers from several limitations like poor penetration of the abnormal tumor microenvironment, relative hypoxia, an unknown endosomal escape mechanism, and relatively complex manufacturing steps for large-scale production. These shortcomings could be resolved by applying adjuvant therapy like vascular normalizing agents and other agents, such as TNF-α and angiotensin-II. Additionally, pH-responsive carbonate apatite (CA) () and strontium sulfite NPs could be employed to ensure quick release of the drugs from the NPs and subsequent endosomal escape of the free drugs at early endosomal stage [
30]. A schematic diagram in shows how fibronectin-coated CA NPs could facilitate specific-integrin-targeted drug delivery to cancer cells.