Pathological angiogenesis, which is mainly induced through vascular endothelial growth factors (VEGF) and their receptors, is involved in many of these vision-impairing ocular disorders, such as age-related macular degeneration (AMD), diabetic retinopathy (DR), and corneal neovascularization.
- gene therapy
- viral vectors
- anti-angiogenesis
- preclinical
- clinical
1. Introduction
Ocular neovascularization is one of the most common causes of moderate and severe vision loss in developed countries. In the healthy eye, VEGF is necessary for the maintenance of retinal pigment epithelium (RPE) and choriocapillaris [1]. As the treatment of the eye possesses several challenges, oral or topical administration of the drug does not achieve therapeutic concentration in the eye [2]. As most of the vision-impairing diseases affect the retina, repeated anti-VEGF injections into the vitreous are currently the most commonly used treatment method in order to inhibit angiogenesis [3]. The treatment with anti-VEGF injections is effective, but it also has disadvantages due to the need for repeated injections, which places a significant burden on the patients and healthcare system and poses a risk of adverse events including inflammation, retinal detachment, and subretinal and vitreous hemorrhage.
Gene therapy with viral vectors for ocular diseases has shown great promise for the future. It provides an alternative treatment for re-occurring injections. Viral vectors are efficient in transducing different tissues and provide long-term treatment options [4]. Successful gene therapy relies on efficient gene delivery to the target cells, which can be achieved by selecting the right delivery system, specific promoter elements, and administration route. An eye is an optimal gene therapy target as it can be easily injected by using only small injection volumes of the virus, still leading to successful expression inside the eye [5][6]. In addition, due to the blood–retina barrier, the gene therapy can be targeted with high titers into the eye, minimizing systemic biodistribution and side effects. Genome editing has brought new possibilities to edit gene expression in the eye. Food and Drug Administration (FDA) approved the first gene transfer treatment for the ocular disease in 2017, where a subretinal delivery of adeno-associated virus serotype 2 (AAV2)-mediated voretigene neparvovecrzyl (Luxturna®,Spark Therapeutics, Inc., Philadephia, PA, USA) is administered for treating inherited biallelic RPE65 mutation-associated retinal dystrophy [7]. The approval of the gene therapy proves that it is a usable tool for treating ocular diseases.
Angiogenesis
Angiogenesis is the growth of blood vessels and is essential for organ growth in the embryo and in the placenta during pregnancy, in the female reproductive system during ovulation and menstruation and wound healing in adults [8]. Angiogenesis is tightly controlled and balanced between proangiogenic and anti-angiogenic factors. One of the most important proangiogenic factors is the VEGF, which induces endothelial cell proliferation, migration, and new vessel formation. The VEGF signals are mediated through tyrosine kinase receptors VEGFR-1, -2, and -3 and co-receptors neuropilins 1 and 2 [9]. VEGF-A is one of the strongest vascular permeability inducers, which expression is increased in hypoxic conditions. Soluble VEGF receptors sVEGFR-1, -2, and -3 function as competitive inhibitors of the binding of VEGFs to the membrane-bound receptors, therefore regulating VEGF signaling [10]. In addition there are other molecules that affect angiogenesis, such as angiopoietin-2 (ANG-2), fibroblast growth factors (FGF), and chemokines, released by hypoxic, inflammatory, or tumor cells [11].
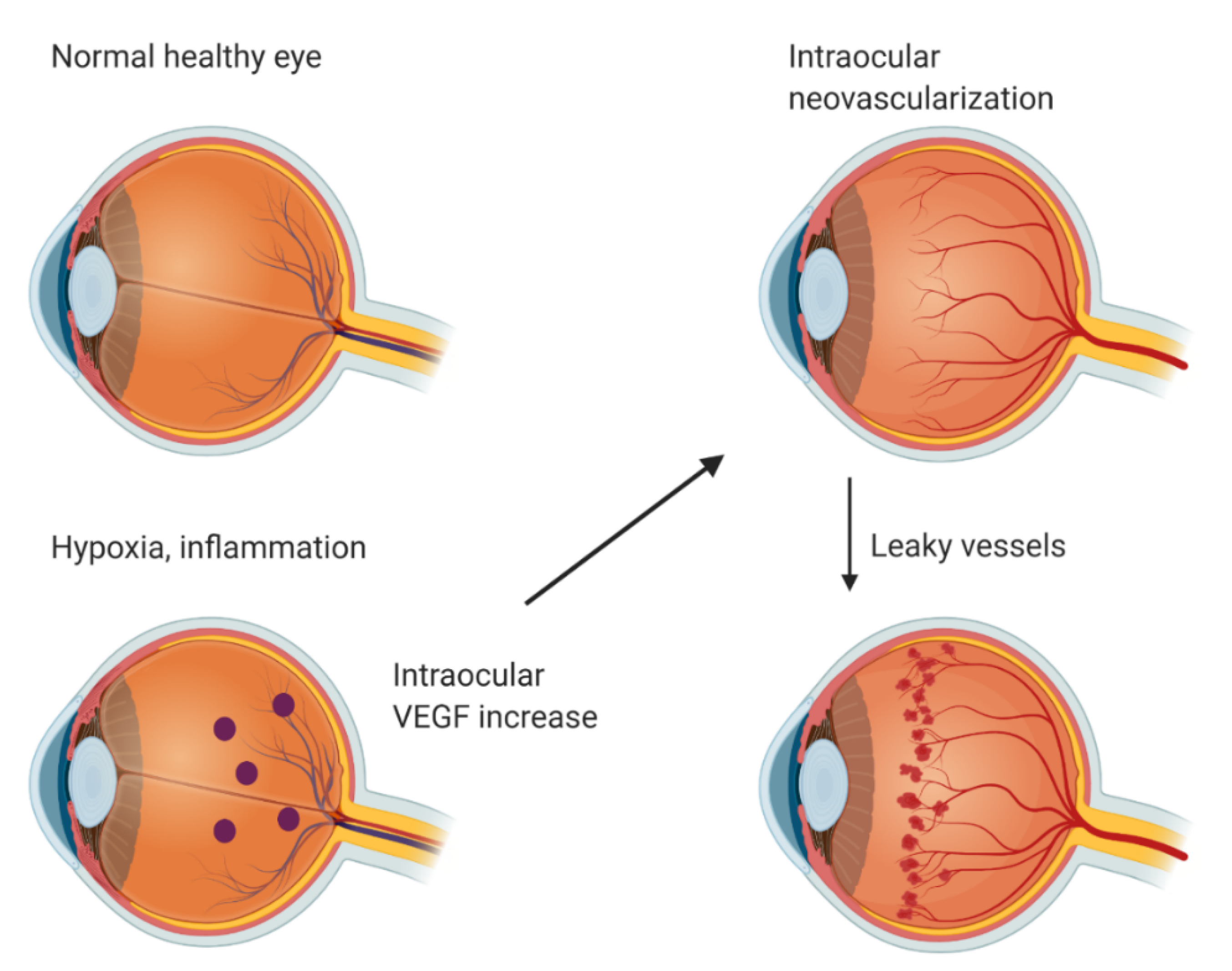
In a normal healthy eye, VEGF is expressed in the retina, choroid-RPE, iris, and conjunctiva [1]. In the retina, VEGF expression is localized in the ganglion, inner nuclear, and RPE cell layers. VEGF is crucial for cellular homeostasis as a neurotrophic and cell survival factor but it is also one of the critical mediators of pathological neovascularization [12]. Intraocular VEGF is upregulated by hypoxia and inflammation (Figure 1). Upregulated VEGF drives neovascularization and increases vascular permeability. This leads to different complications such as leaky vessels, retinal detachment, fibrovascular proliferation, retinal exudation, edema, and ultimately photoreceptor neuron death and blindness. Pathological angiogenesis in the eye includes many diseases—for example, AMD, DR, retinopathy of prematurity (ROP), and corneal neovascularization [13].
Currently available treatment options for ocular neovascularization, especially for wet AMD, include photodynamic therapy (PDT), laser therapy, and anti-VEGF therapies [14]. The use of laser therapy and photodynamic therapy for treatment has declined over time [15]. Laser surgery and PDT have a limited visual improvement potential and, in addition, panretinal photocoagulation laser treatment for DR even has a risk of producing vision loss due to its inherently destructive approach [16][17], although when using newer laser technologies, the risk is lower [18]. The three most commonly used anti-VEGF therapies are aflibercept, ranibizumab, and bevacizumab, which bind to VEGF and prevent their signal transduction through VEGFR [3]. These VEGF inhibitors are injected intravitreally into the eye. Aflibercept is a recombinant fusion protein of human VEGFR-1 and VEGFR-2 binding domains fused with the Fc domain of human IgG1 [19]. Ranibizumab is a recombinant antibody fragment of the humanized anti-VEGF monoclonal antibody. Bevacizumab is a full-length recombinant humanized anti-VEGF monoclonal antibody (IgG). The FDA and European Medicines Agency (EMA) have approved aflibercept and ranibizumab for the treatment of AMD. Bevacizumab has not been approved by the FDA or EMA [20] for ophthalmic use, but its safety and efficacy have been shown in multiple clinical AMD and DR trials. Anti-VEGF therapies have been effective to treat ocular neovascularization [3]. However, intravitreal injections are required monthly, which places a significant burden on the patients and the healthcare system. Injections also pose a risk of serious ocular adverse events, including endophthalmitis, retinal detachment, and subretinal and vitreous hemorrhage.
2. Gene Therapy and Viral Vectors
Gene therapy involves the transfer of nucleic acids into a cell to either correct dysfunctional gene or to provide new cellular functions [21]. Gene therapy can be achieved by the substitution of the altered gene, inhibiting the gene expression or insertion of a new gene [22]. Genes can be transferred by viral or non-viral vectors. Non-viral gene delivery can be achieved by physical or chemical methods. In the physical methods, the permeability of the cell membrane is increased by physical force, and in the chemical methods, natural or synthetic carriers are used. Compared with viral vectors, non-viral vectors are less immunogenic, less toxic, and easily produced. On the other hand, they are less effective at expressing transgenes at the therapeutic level. Viral vectors are more efficient in transducing cells and at inducing long-term gene expression than non-viral vectors [23]. Gene expression time is short with non-viral vectors versus viral vectors, where gene expression can last for weeks, months, or even some years. Viral vectors have limitations—for example, limited transgene capacity or they might cause immune responses. There are many different viral vectors used in gene therapy—for example, adeno-, adeno-associated-, retro-, lenti-, baculo-, herpes simplex-, and Epstein–Barr viruses. Successful gene therapy relies on efficient gene delivery to target cells and long-term gene expression [6]. It can be achieved by selecting the appropriate delivery system, specific promoter elements, and administration route. As a target for gene therapy, the eye has many advantages: eye anatomy is well known, it is easy to access and examine, it is relatively immune-privileged, and study-wise, the other eye can be used as a control.
Adenovirus (Adv) is a non-enveloped double-stranded DNA vector, which can transduce dividing and non-dividing cells [6][24]. Adenovirus can carry large genes of approximately 35 kb [25] and it does not integrate into the host cell genome. There are over 50 different human adenovirus serotypes. Adenoviral vectors transduce different types of cells in the eye [26]. After the intravitreal injection of the adenoviral vector expressing green fluorescent protein (GFP) transduced corneal endothelial cells, trabecular meshwork and iris cells. In subretinally injected eyes, transduction was detected in photoreceptors and RPE cells. In one study, adenoviral-vector-expressing β-galactosidase gene (LacZ) was detected in the nerve cell layer and ganglion cell layer of the retina [27]. In another, adenovirus serotype 5 was shown to transduce efficiently the anterior chamber corneal endothelium, trabecular meshwork, ciliary body, iris and some Müller, and inner nuclear layer (INL) cells [5]. The highest transduction was observed after 7 days of injection. After 1 month of injection, GFP expression was detected mainly in RPE cells. An inflammatory response was observed after 3 and 7 days of injection by an increasing number of F4/80-immunoreactive cells. The time range of how long the transgene is expressed also depends on the Adv serotype [26][28]. It was shown that the Ad35 vector had prolonged transgene expression compared with the Ad5 vector. Transgene expression was detected up to 6 months post Ad35 injection [26] but diminished by 8 months [28]. This is significantly longer than the gene expression seen in animal models after the most often used Ad5 gene delivery. The expression after intravitreal and subretinal Ad5-mediated injection is a couple of weeks [26][28][29]. Adenoviral gene therapy in clinical trials has shown a mild inflammatory response which has been easily managed [30][31].
Adeno-associated virus (AAV) is a single-stranded DNA vector, which can transduce dividing and non-dividing cells [32]. AAV provides long-term gene expression ranging from months to years in preclinical trials [33] and it can integrate into the host cell genome. There is a concerning possibility that integration of the AAV vector might cause insertional mutagenesis, even though the majority of experiments have shown that AAV vectors are safe and potential genotoxic effects can be minimized by vector design [32]. In addition, 3-year follow-up showed detained improvement in best corrected visual acuity (BCVA) after subretinal AAV2-mediated gene therapy in patients with Leber congenital amaurosis type 2 [34]. AAV has many different serotypes, which transduce different cells in the eye. AAV2-expressing GFP has been shown to transduce RPE, the retinal ganglion cell layer (RGCL), and some cells in the INL and the outer plexiform layer (OPL) [5]. After 1 month, GFP expression was also detected in the optic nerve, inner plexiform layer, ciliary body, trabecular meshwork, and iris. After 3 and 6 months of injection, expression was detected in RGCL and INL. In one study, subretinal injection of AAV5 and AAV2 was shown to transduce photoreceptor and RPE cells in the murine retina [35]. AAV5 transduced more photoreceptor cells than AAV2 and expression levels were maintained for up to 31 weeks, which was the latest point tested. The time course for expression was faster with AAV5 than with AAV2. Only minimal to moderate ocular inflammatory responses have been detected after AAV injections into the eye in animal models [33][36] and clinical trials [37][38][39][40][41].
Lentiviruses (LV) and retroviruses are single-stranded RNA vectors [42]. Lentiviruses can transduce dividing and non-dividing cells but retroviruses can transduce only dividing cells. Both virus vectors can integrate stably into the host cell genome. The most commonly used retro- and lentiviral vectors for gene therapy are the human immunodeficiency virus type 1 (HIV-1) and murine Moloney leukemia virus (MLV) [43]. The use of retroviruses for gene therapy has been reduced [6] after a study outcome where four patients developed T cell leukemia 31–68 months after the gamma retroviral gene delivery [44]. As a consequence of the vector integration, proto-oncogenes were activated in all four patients. Lentivirus vectors transduce different cells in the eye. It was shown that lentivirus vector expressing GFP transduce RPE, corneal endothelium, trabecular meshwork, ciliary body, iris pigmented epithelium, anterior chamber, INL, and ocular muscles after intravitreal injection [5]. It was shown that subretinal injection of lentiviral vector expressing GFP transduced RPE [45], choroid, and sclera layers [46]. Inflammatory responses in eyes treated with lentiviral vector injections have been shown to be very moderate or not significant [47][48]. In one study, subretinal lentiviral vectors have shown strong retinal and ocular inflammatory reactions, which resolved over the follow-up period [49]. In a clinical study, it was shown that subretinal injection of the lentiviral vector was well tolerated, with no dose-limiting toxicities, and there was little or no ocular inflammation [50].
Baculoviruses (BV) are double-stranded DNA vectors, which are capable of transferring genes into various mammalian cells without viral replication [51]. Baculoviruses have high transgene capacity and have low cytotoxicity. It has been shown that baculovirus transduces different cells in the eye. Subretinal injection of baculovirus encoding GFP under the CMV promoter (rBV-CMV-GFP) led to GFP expression in RPE cells, which peaked 1–4 days after injection but was not detected after 2 months [52]. When rBV-CMV-GFP was injected intravitreally, GFP expression was detected in the corneal endothelium, the retinal inner nuclear layer, the ganglion cell layer, and the RPE cell layer. After 2 days of subretinal injection, the infiltration of macrophages was observed in the subretinal space and across the retina and after intravitreal injection in the anterior chamber and vitreous. In addition after 3 days, CD11b+ and CD4+ cells were found, but after 8 days, inflammation was almost completely resolved. In another study, intravitreally injected BV-GFP led to GFP expression in the photoreceptor cells and RPE after 6 days of injection [27]. In this study, inflammatory responses were detected in the anterior segment, retina, and the optic nerve head of the eye.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13020219
References
- Kim, I.; Ryan, A.M.; Rohan, R.; Amano, S.; Agular, S.; Miller, J.W.; Adamis, A.P. Constitutive Expression of VEGF, VEGFR-1, and VEGFR-2 in Normal Eyes. Investig. Ophthalmol. 2016, 40, 2115–2121.
- del Amo, E.M.; Urtti, A. Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov. Today 2008, 13, 135–143.
- Wykoff, C.C.; Clark, W.L.; Nielsen, J.S.; Brill, J.V.; Greene, L.S.; Heggen, C.L. Optimizing Anti-VEGF Treatment Outcomes for Patients with Neovascular Age-Related Macular Degeneration. J. Manag. Care Spec. Pharm. 2018, 24, S3–S15.
- Ylä-Herttuala, S.; Bridges, C.; Katz, M.G.; Korpisalo, P. Angiogenic gene therapy in cardiovascular diseases: Dream or vision? Eur. Heart J. 2017, 38, 1365–1371.
- Kalesnykas, G.; Kokki, E.; Alasaarela, L.; Lesch, H.P.; Tuulos, T.; Kinnunen, K.; Uusitalo, H.; Airenne, K.; Yla-Herttuala, S. Comparative Study of Adeno-associated Virus, Adenovirus, Bacu lovirus and Lentivirus Vectors for Gene Therapy of the Eyes. Curr. Gene Ther. 2017, 17, 235–247.
- Solinís, M.Á.; Del Pozo-Rodríguez, A.; Apaolaza, P.S.; Rodríguez-Gascón, A. Treatment of ocular disorders by gene therapy. Eur. J. Pharm. Biopharm. 2015, 95, 331–342.
- Ameri, H. Prospect of retinal gene therapy following commercialization of voretigene neparvovec-rzyl for retinal dystrophy mediated by RPE65 mutation. J. Curr. Ophthalmol. 2018, 30, 1–2.
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004, 56, 549–580.
- Ylä-Herttuala, S.; Rissanen, T.T.; Vajanto, I.; Hartikainen, J. Vascular Endothelial Growth Factors. Biology and Current Status of Clinical Applications in Cardiovascular Medicine. J. Am. Coll. Cardiol. 2007, 49, 1015–1026.
- Vuorio, T.; Jauhiainen, S.; Ylä-Herttuala, S. Pro- and anti-angiogenic therapy and atherosclerosis with special emphasis on vascular endothelial growth factors. Expert Opin. Biol. Ther. 2012, 12, 79–92.
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307.
- Sene, A.; Chin-Yee, D.; Apte, R.S. Seeing through VEGF: Innate and adaptive immunity in pathological angiogenesis in the eye. Trends Mol. Med. 2015, 21, 43–51.
- Liu, C.H.; Wang, Z.; Sun, Y.; Chen, J. Animal models of ocular angiogenesis: From development to pathologies. FASEB J. 2017, 31, 4665–4681.
- Al-Zamil, W.M.; Yassin, S.A. Recent developments in age-related macular degeneration: A review. Clin. Interv. Aging 2017, 12, 1313.
- McLaughlin, M.D.; Hwang, J.C. Trends in Vitreoretinal Procedures for Medicare Beneficiaries, 2000 to 2014. Ophthalmology 2017, 124, 667–673.
- Deschler, E.K.; Sun, J.K.; Silva, P.S. Side-effects and complications of laser treatment in diabetic retinal disease. Semin. Ophthalmol. 2014, 29, 290–300.
- Mason, J.O.; Yunker, J.J.; Vail, R.; Mcgwin, G. Intravitreal bevacizumab (Avastin) prevention of panretinal photocoagulation-induced complications in patients with severe proliferative diabetic retinopathy. Retina 2008, 28, 1319–1324.
- Fijalkowski, N.; Moshfeghi, D.M. New Laser Technologies for Diabetic Retinopathy. Curr. Ophthalmol. Rep. 2013, 1, 134–143.
- Zhao, Y.; Singh, R.P. The role of anti-vascular endothelial growth factor (anti-VEGF) in the management of proliferative diabetic retinopathy. Drugs Context 2018, 7, 212532.
- Avastin|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/avastin#overview-section (accessed on 27 November 2020).
- Cotrim, A.P.; Baum, B.J. Gene Therapy: Some History, Applications, Problems, and Prospects. Toxicol. Pathol. 2008, 36, 97–103.
- Oliveira, A.V.; Rosa da Costa, A.M.; Silva, G.A. Non-viral strategies for ocular gene delivery. Mater. Sci. Eng. C 2017, 77, 1275–1289.
- Rissanen, T.T.; Ylä-Herttuala, S. Current status of cardiovascular gene therapy. Mol. Ther. 2007, 15, 1233–1247.
- Volpers, C.; Kochanek, S. Adenoviral vectors for gene transfer and therapy. J. Gene Med. 2004, 6, S164–S171.
- Suzuki, T.; Sasaki, T.; Yano, K.; Sakurai, F.; Kawabata, K.; Kondoh, M.; Hayakawa, T.; Yagi, K.; Mizuguchi, H. Development of a recombinant adenovirus vector production system free of replication-competent adenovirus by utilizing a packaging size limit of the viral genome. Virus Res. 2011, 158, 154–160.
- Ueyama, K.; Mori, K.; Shoji, T.; Omata, H.; Gehlbach, P.L.; Brough, D.E.; Wei, L.L.; Yoneya, S. Ocular localization and transduction by adenoviral vectors are serotype-dependent and can be modified by inclusion of rgd fiber modifications. PLoS ONE 2014, 9.
- Kinnunen, K.; Kalesnykas, G.; Mähönen, A.J.; Laidinen, S.; Holma, L.; Heikura, T.; Airenne, K.; Uusitalo, H.; Ylä-Herttuala, S. Baculovirus is an efficient vector for the transduction of the eye: Comparison of baculovirus- and adenovirus-mediated intravitreal vascular endothelial growth factor D gene transfer in the rabbit eye. J. Gene Med. 2009, 11, 382–389.
- Hamilton, M.M.; Byrnes, G.A.; Gall, J.G.; Brough, D.E.; Richter King, C.; Wei, L.L. Alternate serotype adenovector provides long-term therapeutic gene expression in the eye. Mol. Vis. 2008, 14, 2535–2546.
- Reichel, M.B.; Ali, R.R.; Thrasher, A.J.; Hunt, D.M.; Bhattacharya, S.S.; Baker, D. Immune responses limit adenovirally mediated gene expression in the adult mouse eye. Gene Ther. 1998, 5, 1038–1046.
- Ildefonso, C.J.; Kong, L.; Leen, A.; Chai, S.J.; Petrochelli, V.; Chintagumpala, M.; Hurwitz, M.Y.; Chévez-Barrios, P.; Hurwitz, R.L. Absence of systemic immune response to adenovectors after intraocular administration to children with retinoblastoma. Mol. Ther. 2010, 18, 1885–1890.
- Campochiaro, P.A.; Nguyen, Q.D.; Shah, S.M.; Klein, M.L.; Holz, E.; Frank, R.N.; Saperstein, D.A.; Gupta, A.; Stout, J.T.; Macko, J.; et al. Adenoviral Vector-Delivered Pigment Epithelium-Derived Factor for Neovascular Age-Related Macular Degeneration: Results of a Phase I Clinical Trial. Hum. Gene Ther. 2006, 17, 167–176.
- Deyle, D.R.; Russell, D.W. Adeno-associated virus vector integration. Curr. Opin. Mol. Ther. 2009, 11, 442–447.
- Liu, Y.; Fortmann, S.D.; Shen, J.; Wielechowski, E.; Tretiakova, A.; Yoo, S.; Kozarsky, K.; Wang, J.; Wilson, J.M.; Campochiaro, P.A. AAV8-antiVEGFfab Ocular Gene Transfer for Neovascular Age-Related Macular Degeneration. Mol. Ther. 2018, 26, 542–549.
- Testa, F.; Maguire, A.M.; Rossi, S.; Pierce, E.A.; Melillo, P.; Marshall, K.; Banfi, S.; Surace, E.M.; Sun, J.; Acerra, C.; et al. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with leber congenital amaurosis type 2. Ophthalmology 2013, 120, 1283–1291.
- Yang, G.S.; Schmidt, M.; Yan, Z.; Lindbloom, J.D.; Harding, T.C.; Donahue, B.A.; Engelhardt, J.F.; Kotin, R.; Davidson, B.L. Virus-Mediated Transduction of Murine Retina with Adeno-Associated Virus: Effects of Viral Capsid and Genome Size. J. Virol. 2002, 76, 7651–7660.
- Ye, G.J.; Conlon, T.; Erger, K.; Sonnentag, P.; Sharma, A.K.; Howard, K.; Knop, D.R.; Chulay, J.D. Safety and Biodistribution Evaluation of rAAV2tYF-CB-hRS1, a Recombinant Adeno-Associated Virus Vector Expressing Retinoschisin, in RS1-Deficient Mice. Hum. Gene Ther. Clin. Dev. 2015, 26, 177–184.
- MacLachlan, T.K.; Lukason, M.; Collins, M.; Munger, R.; Isenberger, E.; Rogers, C.; Malatos, S.; Dufresne, E.; Morris, J.; Calcedo, R.; et al. Preclinical safety evaluation of AAV2-sFLT01 a gene therapy for age-related macular degeneration. Mol. Ther. 2011, 19, 326–334.
- Constable, I.J.; Pierce, C.M.; Lai, C.-M.M.; Magno, A.L.; Degli-Esposti, M.A.; French, M.A.; McAllister, I.L.; Butler, S.; Barone, S.B.; Schwartz, S.D.; et al. Phase 2a Randomized Clinical Trial: Safety and Post Hoc Analysis of Subretinal rAAV.sFLT-1 for Wet Age-related Macular Degeneration. EBioMedicine 2016, 14, 168–175.
- Maguire, A.M.; High, K.A.; Auricchio, A.; Wright, J.F.; Pierce, E.A.; Testa, F.; Mingozzi, F.; Bennicelli, J.L.; Ying, G.S.; Rossi, S.; et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: A phase 1 dose-escalation trial. Lancet 2009, 374, 1597–1605.
- Bennett, J.; Wellman, J.; Marshall, K.A.; McCague, S.; Ashtari, M.; DiStefano-Pappas, J.; Elci, O.U.; Chung, D.C.; Sun, J.; Wright, J.F.; et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: A follow-on phase 1 trial. Lancet 2016, 388, 661–672.
- Cukras, C.; Wiley, H.E.; Jeffrey, B.G.; Sen, H.N.; Turriff, A.; Zeng, Y.; Vijayasarathy, C.; Marangoni, D.; Ziccardi, L.; Kjellstrom, S.; et al. Retinal AAV8-RS1 Gene Therapy for X-Linked Retinoschisis: Initial Findings from a Phase I/IIa Trial by Intravitreal Delivery. Mol. Ther. 2018, 26, 2282–2294.
- Worgall, S.; Crystal, R.G. Gene Therapy. In Principles of Tissue Engineering, 4th ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 657–686. ISBN 9780123983589.
- Vargas, J.E.; Chicaybam, L.; Stein, R.T.; Tanuri, A.; Delgado-Cañedo, A.; Bonamino, M.H. Retroviral vectors and transposons for stable gene therapy: Advances, current challenges and perspectives. J. Transl. Med. 2016, 14, 288.
- Hacein-Bey-Abina, S.; Garrigue, A.; Wang, G.P.; Soulier, J.; Lim, A.; Morillon, E.; Clappier, E.; Caccavelli, L.; Delabesse, E.; Beldjord, K.; et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Investig. 2008, 9, 3132–3142.
- Askou, A.L.; Benckendorff, J.N.E.; Holmgaard, A.; Storm, T.; Aagaard, L.; Bek, T.; Mikkelsen, J.G.; Corydon, T.J. Suppression of Choroidal Neovascularization in Mice by Subretinal Delivery of Multigenic Lentiviral Vectors Encoding Anti-Angiogenic MicroRNAs. Hum. Gene Ther. Methods 2017, 28, 222–233.
- Bemelmans, A.P.; Bonnel, S.; Houhou, L.; Dufour, N.; Nandrot, E.; Helmlinger, D.; Sarkis, C.; Abitbol, M.; Mallet, J. Retinal cell type expression specificity of HIV-1-derived gene transfer vectors upon subretinal injection in the adult rat: Influence of pseudotyping and promoter. J. Gene Med. 2005, 7, 1367–1374.
- Cheng, L.; Chaidhawangul, S.; Wong-Staal, F.; Gilbert, J.; Poeschla, E.; Toyoguchi, M.; El-Bradey, M.H.; Bergeron-Lynn, G.; Soules, K.A.; Freeman, W.R. Human immunodeficiency virus type 2 (HIV-2) vector-mediated in vivo gene transfer into adult rabbit retina. Curr. Eye Res. 2002, 24, 196–201.
- Ikeda, Y.; Yonemitsu, Y.; Miyazaki, M.; Kohno, R.I.; Murakami, Y.; Murata, T.; Goto, Y.; Tabata, T.; Ueda, Y.; Ono, F.; et al. Acute toxicity study of a simian immunodeficiency virus-based lentiviral vector for retinal gene transfer in nonhuman primates. Hum. Gene Ther. 2009, 20, 943–954.
- Matet, A.; Kostic, C.; Bemelmans, A.-P.; Moulin, A.; Rosolen, S.G.; Martin, S.; Mavilio, F.; Amirjanians, V.; Stieger, K.; Lorenz, B.; et al. Evaluation of tolerance to lentiviral LV-RPE65 gene therapy vector after subretinal delivery in non-human primates. Transl. Res. 2017, 188, 40–57.e4.
- Campochiaro, P.A.; Lauer, A.K.; Sohn, E.H.; Mir, T.A.; Naylor, S.; Anderton, M.C.; Kelleher, M.; Harrop, R.; Ellis, S.; Mitrophanous, K.A. Lentiviral Vector Gene Transfer of Endostatin/Angiostatin for Macular Degeneration (GEM) Study. Hum. Gene Ther. 2017.
- Ono, C.; Okamoto, T.; Abe, T.; Matsuura, Y. Baculovirus as a tool for gene delivery and gene therapy. Viruses 2018, 10, 510.
- Haeseleer, F.; Imanishi, Y.; Saperstein, D.A.; Palczewski, K. Gene transfer mediated by recombinant baculovirus into mouse eye. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3294–3300.
