Organochlorine pesticides (OCPs) embody highly lipophilic hazardous chemicals that are being phased out globally. Due to their persistent nature, they are still contaminating the environment, being classified as persistent organic pollutants (POPs). They bioaccumulate through bioconcentration and biomagnification, leading to elevated concentrations at higher trophic levels. Studies show that human long-term exposure to OCPs is correlated with a large panel of common chronic diseases. Due to toxicity concerns, most OCPs are listed as persistent organic pollutants (POPs). Conventionally, separation techniques such as gas chromatography are used to analyze OCPs (e.g., gas chromatography coupled with mass spectrometry (GC/MS) or electron capture detection (GC/ECD)).
- Organochlorine Pesticides
- Surface Enhanced Raman Spectroscopy
1. Introduction
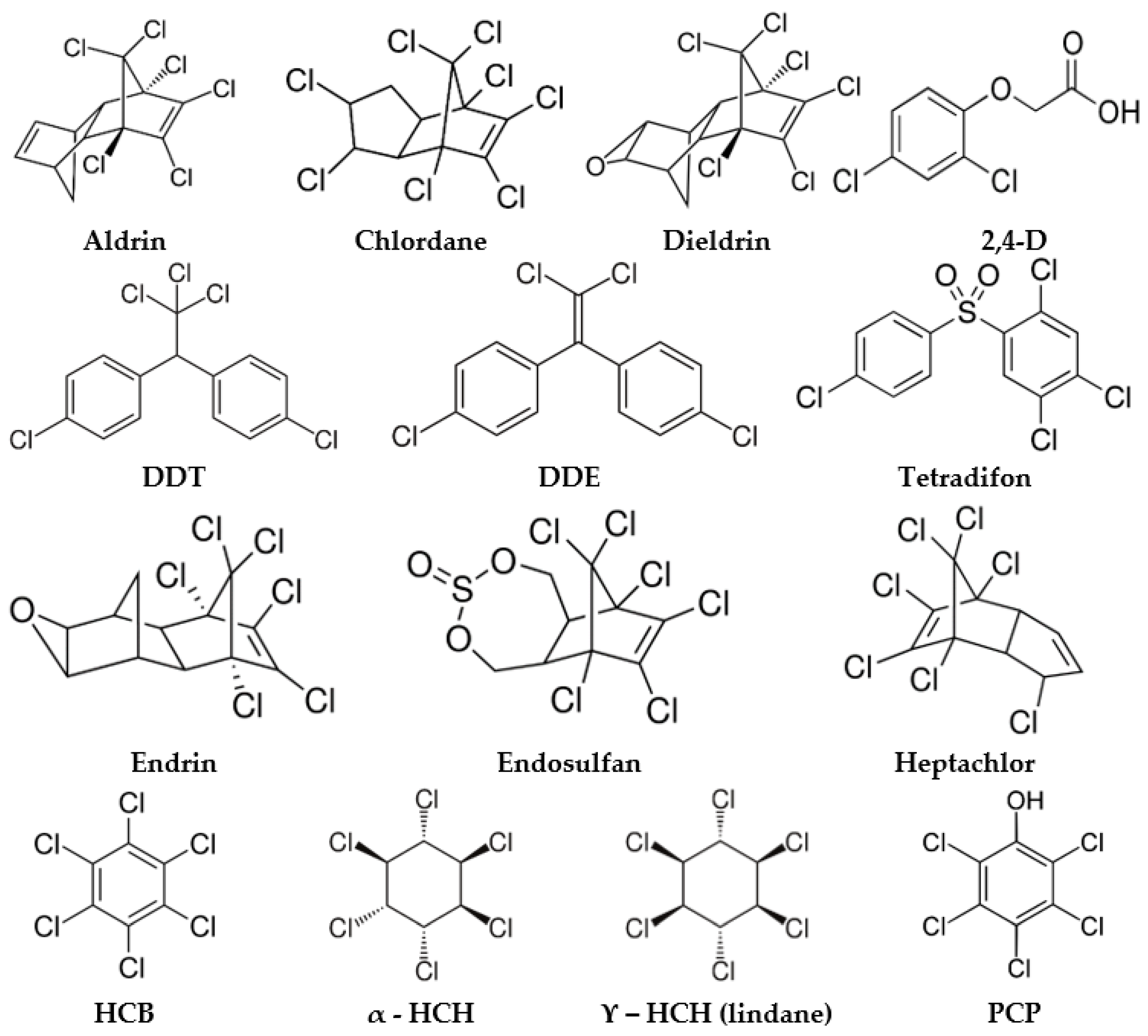
Organochlorine pesticides (OCPs) are synthetic organic molecules that have multiple chlorine atoms in their structure. They were widely used from the 1940s [1] to the 1960s in the United States and Europe mainly to control insect pests by affecting their nervous system [2]. Most OCPs are lipophilic and hydrophobic (Figure 1), and as such, they are very resistant to environmental degradation [3], persisting in the environment for years or decades after usage. This leads to the contamination of groundwater, surface water, food products, air, and soil. An extensive literature review [4] of data from North America, Europe, and Asia on emerging pollutants in water sources identified organochlorine pesticides as one of the most common water pollutants. Due to the great health risks posed by OCPs at environmental concentrations, most of them are included in the group of contaminants known as persistent organic pollutants (POPs).
As the negative impact of pesticide pollution transcends national boundaries, POPs have been regulated by the International Agreement (United Nations Environment Program, UNEP) between 154 signatories of the Stockholm Convention on Persistent Organic Pollutants since 2001. Nine OCPs, namely aldrin, dieldrin, endrin, dichloro-diphenyl-trichloroethane (DDT), chlordane, hexachlorobenzene (HCB), mirex, toxaphene, and heptachlor, were initially listed amongst the 12 initial POPs [5] called the dirty dozen. Since then, chlordecone and hexachlorocyclohexanes (HCH) including lindane (γ-HCH), endosulfan (α and β isomers), dicofol, pentachlorophenol (PCP) and pentachlorobenzene have also been listed [6]. These chemicals are dangerously toxic pollutants, capable of long-range transport, bio-accumulation in human and animal tissues, and bio-magnification in food chains [7].
Many studies keep finding associations between human long-term exposure to OCPs and a large panel of common chronic diseases including cancer (breast, prostate, testicular, kidney, ovarian and uterine cancers), neurodegenerative diseases (Parkinson, Alzheimer, Amyotrophic Lateral Sclerosis), chronic respiratory diseases (asthma and Chronic Obstructive Pulmonary Disorder), diabetes, immune dysfunction, cardiovascular diseases, endocrine disruption, and even harmful reproduction effects [8][9][10]. There are some recent reviews that cover this topic [11][12].
Even if great efforts are made to phase POPs out globally, with obvious progress, they are still found in the environment in regions such as the Arctic [13], thousands of kilometers from any major POP source. Moreover, as novel data are gathered, the list of EPs or environmentally persistent hazardous chemicals is continuously amended. Most recently, in June 2017, the Stockholm Convention established global bans on 16 new POPs [14] including organochlorine pesticides. This translates into financial (expensive equipment) and technical challenges (time-consuming purification processes) regarding the scientific analysis of the samples given that they are conventionally analyzed by chromatographic techniques such as gas chromatography coupled with mass spectrometry (GC/MS) or electron capture detection (GC/ECD) and HPLC-MS [15][16][17]. Even if the detection and quantification of analytes at trace levels is accurate down to ng or pg/L, these techniques can only be used in well-equipped centralized laboratories, once the sample is collected and extensively pre-processed. In this context, accessible new strategies for inexpensive, fast, highly sensitive, and on-site detection of OCPs such as surface enhanced Raman spectroscopy (SERS) are of very high demand. The current review focused on strategies adopted to maximize the SERS signal of OCPs. This can be achieved by increasing the substrate’s performance by increasing the affinity of the analyte for the substrate or by preconcentration. Strategies like concentrating nanoparticles through mechanical traps [18], inducing hot-spots by aggregation or assembly of nanostructures [19] in different-sized oligomers, films, or film patches [20] are all improving the sensitivity of the substrate. Such approaches may even enable the detection of molecules with low affinities toward the metal substrate.
While maintaining their sensitivity, the selectivity of the SERS substrates may be further tuned by functionalizing them with linkers such as diamine [21][22] dithiols [23], bipyridinium dications [24], carbon, and metal-organic-frameworks [25], to name a few. The interaction of these “receptor” molecules with OCPs via covalent, electrostatic, or hydrophobic bonds will be discussed. Moreover, the potential advantages of multiplex analysis and future prospects are also presented.
2. Surface Enhanced Raman Spectroscopy (SERS) Strategies for Organochlorine Pesticide (OCP) Detection
The inelastic scattering of photons by molecules, discovered in 1928 as “a new type of secondary radiation” [26], is known as Raman scattering. This effect is very weak, specifically, there is an inelastic scattering for every ten million elastically scattered photons. As Raman spectroscopy provides the great advantage of a molecular fingerprint (in particular, the molecule’s vibrational structure), it was fortunate that in 1974, researchers developed discovered methods to amplify the weak Raman signal [27]. This is when SERS emerged. The SERS effect denotes a strong increase (several orders of magnitude) in the Raman signal of a molecule, induced with the help of a special substrate, typically represented by metallic nanostructures.
The enhancement of the Raman signal by the metal substrate is explained mainly by an electromagnetic mechanism cumulated or not with a chemical one [28][29]. At a certain resonant frequency, the interaction of electromagnetic radiation with metal nanostructures leads to collective oscillations of the conduction electrons (i.e., the excitation of surface plasmons (plasmon resonance)). One of the main consequences of this resonant plasmon excitation is the strong enhancement of the electromagnetic near fields at the metal surface. It is this resonantly enhanced near field that is the main contributor to the Raman signal enhancement in SERS. Since near fields decay exponentially away from the surface, it has been established that it is critical for the molecule to be in close proximity of the metallic surface (furthermost 10 nm) [28] to increase the Raman signal. The enhancement is maximized for molecules in direct contact with the surface, and it decreases with the increase in distance between the substrate and the analyte. By matching the surface plasmon resonances (localized or propagative) to the excitation laser, which can be done by material, size, and shape adjustments, the SERS efficiency of a substrate can be maximized at the desired wavelength. To extend SERS effects into the deep-UV and benefit at the same time from the resonance Raman effect (i.e., matching molecular electronic transitions), aluminum SERS substrates have been developed [30].
The SERS enhancement based on the electromagnetic mechanism applies to all molecules and leads to enhancement factors (EF) as high as 106–108. The chemical mechanism, on the other hand, is based on charge transfer interactions that take place between molecules and the metal surface. This requires the molecule to be chemically adsorbed on the surface of the metal substrate, making this mechanism analyte-dependent and site-specific. Molecules can be adsorbed on the surface either through physisorption (van der Waals forces) or chemisorption (chemical bonds such as covalent or electrostatic interactions).
Since it provides a broad range of advantages, SERS represents a valuable analytical tool for the detection of pesticides. Among these, the high sensitivity and the generated vibrational fingerprint of molecules are highly valued since OCPs are found in trace amounts in rather complex matrices of the environmental samples and they can have multiple stereoisomeric configurations. Moreover, the analyses are fast (given that there is no need for thorough purification processes), the equipment is not very costly, and it is easy to use. Additionally, there are portable Raman spectrometers for on-site analysis.
As every analytical technique, it has also its drawbacks, certain concerns in the SERS analysis (of pesticides) involving selectivity, sensibility, reproducibility, portability, quantification, and nonspecific binding. These challenges have been the subject of multiple comprehensive reviews [31][32][33], consequently they will not be reconsidered in detail. Bernat et al. [34] presented limitations like selectivity, reproducibility, and nonspecific binding, along with some possible solutions. Reviews regarding different spectroscopic techniques (SERS, SPR, and fluorescence) for detecting POPs [35] or pesticide residues in foods [36][37] can also be found in the literature. Some recent book chapters have addressed more general matters like usage, sensing (exploiting biofunctionalized nanomaterials), and removal of different classes of pesticides (organochlorine, organophosphate, carbamate, pyrethroids and others) [38] or development of optical (including SERS) and electrochemical sensors for pesticide detection [39].[40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58]
OCPs are hardly mentioned in these works as their SERS spectrum is more troublesome to be obtained because of their highly hydrophobic structures (Figure 1) and low affinity toward the SERS substrates. Additionally, the important progress made in the last few years regarding their analysis has built the momentum of a more comprehensive discussion and review on the SERS detection strategies of OCPs, as summarized in Table 1.
Table 1. Surface enhanced Raman spectroscopy (SERS) strategies for Organochlorine pesticide (OCP) detection.
| Strategy | Analyte | SERS Substrate/Method | LOD | EF | Metal | Laser (nm) | Incubation Time | Ref. |
|---|---|---|---|---|---|---|---|---|
| Increasing the substrate’s performance | 2,4-D | Au nanorods and Ag nanocubes | - | - | Au, Ag | 632.8 | 1 h | [40] |
| vertically ordered arrays of Ag nanorod bundles |
61.9 nM | 1.4 × 108 | Ag + Au | 633 | hours | [41] | ||
| DDT | void@AuNPs@SiO2 microporous capsules | - | - | Au | 785 | 15 min | [42] | |
| AgNP@composite agarose gels | - | - | Ag | 785 | 2 h | [18] | ||
| AuNP array fabricated by laser annealing of gold film | - | - | Au | 785 | - | [43] | ||
| AgNPs prepared by self-assembly/ in situ growing method |
- | 3.5 × 106 | Ag | 785 | <1 min | [44] | ||
| AgNPs sheet | - | - | Ag | 632.8 | 20 min | [45] | ||
| Endosulfan | AuNPs | - | - | Au | 785 | - | [46] | |
| HCB | 3D Ag F-NPs | - | - | Ag | 785 | 30 min | [47] | |
| Lindane | chestnut-like Au nanocrystals-built film | 34.38 nM | > 107 | Au | 785 | 8h | [19] | |
| concave trisoctahedral and calyptriform Au nanocrystals-built films | 0.1 µM | 107 | Au | 785 | 8h | [48] | ||
| AgNPs sheet | 0.3 µM | - | Ag | 632.8 | 20 min | [45] | ||
| PCP | MoO2nanodumbbells | 0.1 µM | 3.75 × 106 | MoO2 | 532.8 | 20 min | [49] | |
| Fe3O4xAgNPs@pNIPAM | 1 nM | - | Ag | 785 | 2 h | [50] | ||
| >100 pesticides |
electrochemically roughened silver oxide SERS sensor | - | - | Ag | 785 | - | [51] | |
| Increasing the affinity of the analyte | Aldrin | AgNPs@ α, ω-aliphatic diamines | 13.7 nM | 2 × 104 | Ag | 514.5 | - | [21] |
| AuNPs/AgNPs@alkyl dithiols | 0.12 µM | - | Au, Ag | 785 | 10 min | [23] | ||
| AgNPs clusters by α, ω-aliphatic diamines | 10 nM | - | Ag | 785 514.5 |
- | [22] | ||
| flower like AgNPs@ diquat/lucigenin | - | - | Ag | 532 | 5 min | [52] | ||
| Dieldrin | AuNPs/AgNPs@alkyl dithiols | 0.82 µM | - | Au, Ag | 785 | 10 min | [23] | |
| op’-DDT | flower like AgNPs@ diquat/lucigenin | - | - | Ag | 532 | 5 min | [52] | |
| pp’-DDE | flower like AgNPs@ diquat/lucigenin | - | - | Ag | 532 | 5 min | [52] | |
| Endosulfan (α) | AuNPs/AgNPs@alkyl dithiols | 0.41 µM | - | Au, Ag | 785 | 10 min | [23] | |
| Endosulfan (α, β) | AgNP clusters by α, ω-aliphatic diamines | 10nM | - | Ag | 785 514.5 |
- | [22] | |
| Endosulfan | AgNP@ bis-acridinium lucigenin | 49.15 nM | - | Ag | 785 | - | [24] | |
| Endosulfan (α, β) | flower like AgNPs@diquat/lucigenin | - | - | Ag | 532 | 5 min | [52] | |
| HCH | Au nanosheets built hollow sub-microcubes@4-MPBA | 1.03 nM | - | Au | 785 | 4h | [53] | |
| HCH (α, ϒ) | urchin-like Au–Ag nanocrystals@ porous zeolite imidazole framework | 15.15 nM | 3 × 107 | Au + Ag | 785 | 10 h | [25] | |
| HCH (α, β) | flower like AgNPs@diquat/lucigenin | - | - | Ag | 532 | 5 min | [52] | |
| HCH (ϒ) | AuNPs/AgNPs@alkyl dithiols | 3.53 µM | - | Au, Ag | 785 | 10 min | [23] | |
| Heptachlor | flower like AgNPs@ diquat/lucigenin | - | - | Ag | 532 | 5 min | [52] | |
| PCP | AgNPs aggregates@cysteamine SAM | 0.20 μM | - | Ag | 785 | 3 h | [54] | |
| AuNPs@cysteamine | 1 nM | 5.7 × 105 | Au | 785 | - | [55] | ||
| nanoporous Ag coating@cysteamine | 6.4 nM | 3.7 × 105 | Ag | 785 | 5 h | [56] | ||
| Fe3O4@carbon@AgNPs core–shell microspheres | 1 pM | - | Ag | 633 | 1 h | [57] | ||
| Tetradifon | flower like AgNPs@ diquat/lucigenin | - | - | Ag | 532 | 5 min | [52] | |
| Preconcentration | Chlordane | citrate coated AuNPs/rolling method and prediction model | 1 ppm | - | Au | 780 | - | [58] |
| PCP | nanoporous Ag coating modified by cysteamine | 6.4 nM | 3.7 ×105 | Ag | 785 | 5 h | [56] |
This entry is adapted from the peer-reviewed paper 10.3390/nano11020304
References
- Kutz, F.W.; Wood, P.H.; Bottimore, D.P. Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue. Rev. Environ. Contam. Toxicol. 1991, 120, 1–82.
- James, R.R.; Xu, J. Mechanisms by which pesticides affect insect immunity. J. Invertebr. Pathol. 2012, 109, 175–182.
- Idowu, G.; Aiyesanmi, A.; Owolabi, B.J. Organochlorine pesticide residue levels in river and sediment from cocoa producing areas of Ondo State central district, Nigeria. J. Environ. Chem. Ecotoxicol. 2013, 5, 242–249.
- Murray, K.E.; Thomas, S.M.; Bodour, A.A. Prioritizing research for trace pollutants and emerging contaminants in the freshwater environment. Environ. Pollut. 2010, 158, 3462–3471.
- The 12 Initial POPs under the Stockholm Convention. Available online: http://chm.pops.int/TheConvention/ThePOPs/The12InitialPOPs/tabid/296/Default.aspx (accessed on 15 December 2020).
- All POPs Listed in the Stockholm Convention. Available online: http://chm.pops.int/TheConvention/ThePOPs/AllPOPs/tabid/2509/Default.aspx (accessed on 15 December 2020).
- Hu, G.; Dai, J.; Mai, B.; Luo, X.; Cao, H.; Wang, J.; Li, F.; Xu, M. Concentrations and Accumulation Features of Organochlorine Pesticides in the Baiyangdian Lake Freshwater Food Web of North China. Arch. Environ. Contam. Toxicol. 2010, 58, 700–710.
- Wang, Y.; Guo, Y.; Hu, Y.; Sun, Y.; Xu, D. Endosulfan triggers epithelial-mesenchymal transition via PTP4A3-mediated TGF-β signaling pathway in prostate cancer cells. Sci. Total Environ. 2020, 731, 139234.
- Parrón, T.; Requena, M.; Hernández, A.F.; Alarcón, R. Association between environmental exposure to pesticides and neurodegenerative diseases. Toxicol. Appl. Pharmacol. 2011, 256, 379–385.
- Hernández, A.F.; Parrón, T.; Alarcón, R. Pesticides and asthma. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 90–96.
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Zou, W.; Tong, W.; Hong, H. Persistent Organic Pollutants in Food: Contamination Sources, Health Effects and Detection Methods. Int. J. Environ. Res. Public Health 2019, 16, 4361.
- Taiwo, A.M. A review of environmental and health effects of organochlorine pesticide residues in Africa. Chemosphere 2019, 220, 1126–1140.
- Weber, J.; Halsall, C.J.; Muir, D.; Teixeira, C.; Small, J.; Solomon, K.; Hermanson, M.; Hung, H.; Bidleman, T. Endosulfan, a global pesticide: A review of its fate in the environment and occurrence in the Arctic. Sci. Total Environ. 2010, 408, 2966–2984.
- The New POPs under the Stockholm Convention. Available online: http://www.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx (accessed on 15 December 2020).
- Xu, X.; Yang, H.; Li, Q.; Yang, B.; Wang, X.; Lee, F.S.C. Residues of organochlorine pesticides in near shore waters of LaiZhou Bay and JiaoZhou Bay, Shandong Peninsula, China. Chemosphere 2007, 68, 126–139.
- Ali, M.; Kazmi, A.A.; Ahmed, N. Study on effects of temperature, moisture and pH in degradation and degradation kinetics of aldrin, endosulfan, lindane pesticides during full-scale continuous rotary drum composting. Chemosphere 2014, 102, 68–75.
- Guillén, D.; Ginebreda, A.; Farré, M.; Darbra, R.M.; Petrovic, M.; Gros, M.; Barceló, D. Prioritization of chemicals in the aquatic environment based on risk assessment: Analytical, modeling and regulatory perspective. Sci. Total Environ. 2012, 440, 236–252.
- Aldeanueva-Potel, P.; Faoucher, E.; Alvarez-Puebla, R.A.; Liz-Marzán, L.M.; Brust, M. Recyclable Molecular Trapping and SERS Detection in Silver-Loaded Agarose Gels with Dynamic Hot Spots. Anal. Chem. 2009, 81, 9233–9238.
- Zhou, X.; Zhao, Q.; Liu, G.; Zhang, H.; Li, Y.; Cai, W. Kinetically-Controlled Growth of Chestnut-Like Au Nanocrystals with High-Density Tips and Their High SERS Performances on Organochlorine Pesticides. Nanomaterials 2018, 8, 560.
- Farcau, C.; Sangeetha, N.M.; Decorde, N.; Astilean, S.; Ressier, L. Microarrays of gold nanoparticle clusters fabricated by Stop&Go convective self-assembly for SERS-based sensor chips. Nanoscale 2012, 4, 7870–7877.
- Guerrini, L.; Izquierdo-Lorenzo, I.; Garcia-Ramos, J.V.; Domingo, C.; Sanchez-Cortes, S. Self-assembly of α,ω-aliphatic diamines on Ag nanoparticles as an effective localized surface plasmon nanosensor based in interparticle hot spots. Phys. Chem. Chem. Phys. 2009, 11, 7363–7371.
- Guerrini, L.; Izquierdo Lorenzo, I.; Rodriguez-Oliveros, R.; Sánchez-Gil, J.; Sanchez-Cortes, S.; Garcia-Ramos, J.; Domingo, C. α,ω-Aliphatic Diamines as Molecular Linkers for Engineering Ag Nanoparticle Clusters: Tuning of the Interparticle Distance and Sensing Application. Plasmonics 2010, 5, 273–286.
- Kubackova, J.; Fabriciova, G.; Miskovsky, P.; Jancura, D.; Sanchez-Cortes, S. Sensitive Surface-Enhanced Raman Spectroscopy (SERS) Detection of Organochlorine Pesticides by Alkyl Dithiol-Functionalized Metal Nanoparticles-Induced Plasmonic Hot Spots. Anal. Chem. 2015, 87, 663–669.
- Guerrini, L.; Aliaga, A.; Cárcamo-Vega, J.; Gómez-Jeria, J.-S.; Sanchez-Cortes, S.; Vallette, M.; García-Ramos, J. V Functionalization of Ag nanoparticles with the bis-acridinium lucigenin as a chemical assembler in the detection of persistent organic pollutants by surface-enhanced Raman scattering. Anal. Chim. Acta 2008, 624, 286–293.
- Zhou, X.; Liu, G.; Zhang, H.; Li, Y.; Cai, W. Porous zeolite imidazole framework-wrapped urchin-like Au-Ag nanocrystals for SERS detection of trace hexachlorocyclohexane pesticides via efficient enrichment. J. Hazard. Mater. 2019, 368, 429–435.
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502.
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166.
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57.
- Ding, S.-Y.; You, E.-M.; Tian, Z.-Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076.
- Sigle, D.O.; Perkins, E.; Baumberg, J.J.; Mahajan, S. Reproducible Deep-UV SERRS on Aluminum Nanovoids. J. Phys. Chem. Lett. 2013, 4, 1449–1452.
- Li, D.-W.; Zhai, W.-L.; Li, Y.-T.; Long, Y.-T. Recent progress in surface enhanced Raman spectroscopy for the detection of environmental pollutants. Microchim. Acta 2014, 181, 23–43.
- Pang, S.; Yang, T.; He, L. Review of surface enhanced Raman spectroscopic (SERS) detection of synthetic chemical pesticides. TrAC Trends Anal. Chem. 2016, 85, 73–82.
- Ren, B.; Pérez-Jiménez, A.; Lyu, D.; Lu, Z.; Liu, G. Surface-enhanced Raman spectroscopy: Benefits, trade-offs and future developments. Chem. Sci. 2020, 11.
- Bernat, A.; Samiwala, M.; Albo, J.; Jiang, X.; Rao, Q. Challenges in SERS-based pesticide detection and plausible solutions. J. Agric. Food Chem. 2019, 67, 12341–12347.
- Wang, L.; Pang, S.; Zhou, G. Recent Advances in Spectroscopy Technology for Trace Analysis of Persistent Organic Pollutants. Appl. Sci. 2019, 9, 3439.
- Xu, M.-L.; Gao, Y.; Han, X.X.; Zhao, B. Detection of Pesticide Residues in Food Using Surface-Enhanced Raman Spectroscopy: A Review. J. Agric. Food Chem. 2017, 65, 6719–6726.
- Pilot, R. SERS detection of food contaminants by means of portable Raman instruments. J. Raman Spectrosc. 2018, 49.
- Mehta, J.; Kumar, R.; Dhaka, S.; Deep, A. Biofunctionalized Nanostructured Materials for Sensing of Pesticides. In Nanosensors for Environmental Applications; Kumar Tuteja, S., Arora, D., Dilbaghi, N., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Germany, 2020; pp. 29–86. ISBN 978-3-030-38101-1.
- Kalyani, N.; Goel, S.; Jaiswal, S. Point-of-Care Sensors for On-Site Detection of Pesticides. In Nanosensors for Environmental Applications; Kumar Tuteja, S., Arora, D., Dilbaghi, N., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Germany, 2020; pp. 197–224. ISBN 978-3-030-38101-1.
- Santos Costa, J.C.; Ando, R.A.; Sant’Ana, A.C.; Rossi, L.M.; Santos, P.S.; Temperini, M.L.A.; Corio, P. High performance gold nanorods and silver nanocubes in surface-enhanced Raman spectroscopy of pesticides. Phys. Chem. Chem. Phys. 2009, 11, 7491–7498.
- Zhu, C.; Meng, G.; Zheng, P.; Huang, Q.; Li, Z.; Hu, X.; Wang, X.; Huang, Z.; Li, F.; Wu, N. A Hierarchically Ordered Array of Silver-Nanorod Bundles for Surface-Enhanced Raman Scattering Detection of Phenolic Pollutants. Adv. Mater. 2016, 28, 4871–4876.
- Mariño-Lopez, A.; Sousa-Castillo, A.; Blanco-Formoso, M.; Furini, L.N.; Rodríguez-Lorenzo, L.; Pazos-Perez, N.; Guerrini, L.; Pérez-Lorenzo, M.; Correa-Duarte, M.A.; Alvarez-Puebla, R.A. Microporous plasmonic capsules as stable molecular sieves for direct SERS quantification of small pollutants in natural waters. Chem. Nanomater. Energy Biol. More 2019, 5, 46–50.
- Nedyalkov, N.; Nikov, R.; Nikov, R.; Nikolov, A.; Atanasov, P.; Nakajima, Y.; Terakawa, M.; Sawczak, M.; Grochowska, K.; Sliwinski, G. Gold nanostructures for detection of pesticides, nitrates and drugs using Surface Enhanced Raman spectroscopy. In Proceedings of the 19th International Conference and School on Quantum Electronics: Laser Physics and Applications; Dreischuh, T., Gateva, S., Daskalova, A., Serafetinides, A., Eds.; SPIE: Washington, DC, USA, 2017; Volume 10226, pp. 85–92.
- Cai, L.; Deng, Z.; Dong, J.; Song, S.; Wang, Y.; Chen, X. Fabrication of Non-Woven Fabric-Based SERS Substrate for Direct Detection of Pesticide Residues in Fruits. J. Anal. Test. 2017, 1, 322–329.
- Chi, H.; Wang, C.; Wang, Z.; Zhu, H.; Mesias, V.S.D.; Dai, X.; Chen, Q.; Liu, W.; Huang, J. Highly reusable nanoporous silver sheet for sensitive SERS detection of pesticides. Analyst 2020, 145, 5158–5165.
- Hernández-Castillo, M.I.; Zaca-Morán, O.; Zaca-Morán, P.; Orduña-Diaz, A.; Delgado-Macuil, R.; Rojas-López, M. Surface-enhanced Raman scattering of the adsorption of pesticide endosulfan on gold nanoparticles. J. Environ. Sci. Health. B 2015, 50, 584–589.
- Gong, T.; Huang, Y.; Wei, Z.; Huang, W.; Wei, X.; Zhang, X. Magnetic assembled 3D SERS substrate for sensitive detection of pesticide residue in soil. Nanotechnology 2020, 31, 1–8.
- Zhou, X.; Zhao, Q.; Liu, G.; Zhang, H.; Li, Y.; Cai, W. Temperature regulation growth of Au nanocrystals: From concave trisoctahedron to dendritic structures and their ultrasensitive SERS-based detection of lindane. J. Mater. Chem. C 2017, 5, 10399–10405.
- Zhang, Q.; Li, X.; Ma, Q.; Zhang, Q.; Bai, H.; Yi, W.; Liu, J.; Han, J.; Xi, G. A metallic molybdenum dioxide with high stability for surface enhanced Raman spectroscopy. Nat. Commun. 2017, 8, 1–9.
- Contreras-Cáceres, R.; Abalde-Cela, S.; Guardia-Girós, P.; Fernández-Barbero, A.; Pérez-Juste, J.; Alvarez-Puebla, R.A.; Liz-Marzán, L.M. Multifunctional Microgel Magnetic/Optical Traps for SERS Ultradetection. Langmuir 2011, 27, 4520–4525.
- Spencer, K.; Clauson, S.; Spencer, S.; Sylvia, J.; Vallejos, Q.; Quandt, S.; Arcury, T. Development of a Fieldable Rapid Pesticide Exposure Analysis Sensing System. Proc. SPIE Int. Soc. Opt. Eng. 2010, 7673.
- Zhang, D.; Liang, P.; Yu, Z.; Xia, J.; Ni, D.; Wang, D.; Zhou, Y.; Cao, Y.; Chen, J.; Chen, J.; et al. Self-assembled “bridge” substance for organochlorine pesticides detection in solution based on Surface Enhanced Raman Scattering. J. Hazard. Mater. 2020, 382, 121023.
- Zhou, X.; Zhao, Q.; Liu, G.; Cai, W. 4-Mercaptophenylboronic Acid modified Au Nanosheets-built Hollow Sub-microcubes for Active Capture and Ultrasensitive SERS-based Detection of Hexachlorocyclohexane Pesticides. Sens. Actuators B Chem. 2019, 293.
- Jiang, X.; Yang, M.; Meng, Y.; Jiang, W.; Zhan, J. Cysteamine-Modified Silver Nanoparticle Aggregates for Quantitative SERS Sensing of Pentachlorophenol with a Portable Raman Spectrometer. ACS Appl. Mater. Interfaces 2013, 5, 6902–6908.
- Ma, Q.; Zhang, H.; Liu, W.; Ge, J.; Wu, J.; Wang, S.; Wang, P. Surface-enhanced Raman scattering substrate based on cysteamine-modified gold nanoparticle aggregation for highly sensitive pentachlorophenol detection. RSC Adv. 2016, 6, 85285–85292.
- Bian, W.; Zhu, S.; Qi, M.; Xiao, L.; Liu, Z.; Zhan, J. Electrostatic-driven solid phase microextraction coupled with surface enhanced Raman spectroscopy for rapid analysis of pentachlorophenol. Anal. Methods 2017, 9, 459–464.
- An, Q.; Zhang, P.; Li, J.-M.; Ma, W.-F.; Guo, J.; Hu, J.; Wang, C.C. Silver-coated magnetite–carbon core–shell microspheres as substrate-enhanced SERS probes for detection of trace persistent organic pollutants. Nanoscale 2012, 4, 5210–5216.
- Qu, Y.; He, L. Development of a facile rolling method to amplify an analyte’s weak SERS activity and its application for chlordane detection. Anal. Methods 2020, 12, 433–439.
