Cancer treatment and therapy has made significant leaps and bounds in these past decades. However, there are still cases where surgical removal is impossible, metastases are challenging and chemotherapy and radiotherapy pose severe side effects. Therefore, the need to find more effective and specific treatments still exists. One of the ways is through the utilization of drug delivery agents (DDA) based on nanomaterials. In 2001, mesoporous silica nanoparticles (MSNs) were first used as DDA and have gained considerable attention in this field. The popularity of MSNs is due to their unique properties such as tunable particle and pore size, high surface area and pore volume, easy functionalization and surface modification, high stability and their capability to efficiently entrap cargo molecules. This review describes the latest advancement of MSNs as DDA for cancer treatment. We focus on the fabrication of MSNs, the challenges in DDA development and how MSNs address the problems through development of smart DDA using MSNs. Besides that, MSNs have also been applied as a multifunctional DDA where they can serve in both diagnostic and treatment of cancer. Overall, we argue MSNs provide a bright future for both the diagnosis and treatment of cancer.
- mesoporous silica nanoparticles
- drug delivery
- cancer
1. Introduction
Mesoporous silica nanoparticles (MSNs) can be defined as solid materials that contain pores. The terms mesoporous in MSNs referred to the pore size. According to the International Union of Pure and Applied Chemistry (IUPAC), porous materials can be classified as microporous, mesoporous or macroporous. This is based on pore diameter where less than 2, between 2 and 50 nm and larger than 50 nm belong to a microporous, mesoporous and macroporous category, respectively. MSNs are very popular across various fields due to their properties such as tunable particle and pore size, high stability and rigid framework, large surface area and high pore volume, easy functionalization and simple fabrication[1].
To understand the attraction of MSNs, we should understand their history. The first mesoporous silica material, M41S, was discovered in the 1990s by a researcher in the Mobil Oil company. Like many breakthroughs, the discovery of mesoporous silica was accidental. M41S exhibited almost non-Brønsted acidity, thin amorphous walls, high surface area, large pore volumes and adjustable pore sizes [2][3]. In M41S, there are three main members, namely, MCM-41, MCM-48 and MCM-50. These can be differentiated through their pore geometry where MCM-41 shows hexagonal pore structure, MCM-48 has cubic mesostructured gyroidic phase Ia3d, and MCM-50 exhibits lamellar geometry that consists of silicate or porous aluminosilicate layers separated by surfactant layers[4]. Among these three, MCM-41 is the most widely studied as MCM-48 and MCM-50 are difficult to obtain and thermally unstable[3]. Since then, other types of MSNs have been discovered, for example, folded sheet mesoporous material-16 (FSM-16), Santa Barbara Amorphous family (SBA-n), Fudan University material (FDU), Korea Advanced Institute of Science and Technology (KIT), hollow MSNs and others[5].
2. MSNs as Smart Drug Delivery Agent
The properties of MSNs such as high surface area, biocompatibility, easy functionalization and others make them favorable to be applied as a drug delivery agent. The high surface area allowed a high concentration of drugs to be incorporated, but premature release prior to reaching the target is a definite concern. However, due to the presence of pores in the MSNs, the drug can be loaded into the pores, and the pore opening can be closed with other molecules. To make the MSNs more effective carrier, the gate of the pore can be opened through a specific stimulus. Besides that, these MSNs can also be a multifunctional carrier, and this will be explained in the latter section. In this section, we will discuss and focus on studies that have reported on the production of MSNs as drug delivery agents and their evolution from simple passive targeting of drug delivery to within stimulus-responsive drug delivery methods.
2.1. Passive Targeting
The term passive targeting can be defined as the accumulation of nanoparticles in solid tumors. The foundation of passive targeting was discovered by Matsumura and Meda around 1986. They found two key observations to passive targeting: (1) spontaneous accumulation of drug carrier in areas of solid tumors with leaky vascular and (2) retention of the carrier due to compromise lymphatic drainage. With these two observations, the concept of enhancing permeability and retention (EPR) effect was formed[6]. To further understand the basis of the EPR effect, we must first understand the pathophysiology of the tumor. Solid tumors grow at a rapid rate, and this comes with high nutrients and oxygen demand. Thus, new blood vessels and neovasculature were formed, and this is term as angiogenesis. These new blood vessels often exhibit disorganized course, irregular, discontinuous epithelium and structurally different from healthy vessels. Due to this, the nanoparticles can leak between the gaps and enter the tumor. This stage is referring to the enhanced permeability of the EPR effect. It should be noted that solid tumor has poor lymphatic drainage. Molecules smaller than 4 nm can diffuse back to the bloodstream, but the nanoparticles are impeded due to their larger particle size, thus retain in the solid tumor. This part refers to the retention of the EPR effect[6][7].
For MSNs to be used as a drug delivery agent through passive targeting, there are several parameters that must be controlled, which are the particle size, particle shape and surface properties. The particle size must be larger than the renal clearance level but small enough to be able to diffuse to the tumor cell through the leaky vessel, which is approximately between 50 nm to 300 nm. A previous study showed the non-spherical nanoparticles could reduce phagocytosis, which leads to longer circulation. However, it is difficult to conclude that particle size is the main variable as there are many techniques and materials used to produce MSNs. Therefore, particle size may not be the main parameters that need to be controlled to achieve a good EPR effect[6]. Surface properties of MSNs are the most important aspect for a good EPR effect. With surface modification, the MSNs can avoid the reticuloendothelial system (RES), thus preventing clearance. Polyethylene glycol (PEG) was found to be commonly functionalized with MSNs as it can reduce RES uptake and improve the overall stability of MSNs, which can help to enhance the EPR effect [8][9]. Although this passive targeting through the EPR effect is a good way to deliver drugs, it still poses various limitations. The mechanism of nanoparticle entrance to the tumor is more complex, and this EPR effect is pronounced in small animal xenograft tumor models, which are used in the in vivo study. Although this EPR effect occurs in humans, it varies greatly between a person and tumor type[10]. Even for the same tumor, the vascular permeability and lymphatic drainage differ for each area. This heterogeneity will limit the even distribution of carriers, thus lower the therapeutic effect[6]. Therefore, a better drug delivery agent is needed to overcome this issue by passive targeting.
2.2. Active Targeting
Active targeting is employed to overcome some of the problems posed by passive targeting. This is achieved through exploiting the tumor cell-specific receptors. On tumor cells, a receptor is highly expressed. Thus, it is a sensible target to achieve active targeting. By decorating or functionalizing the MSNs surface with a targeting ligand which can interact with the receptors, the uptake and EPR effect can be enhanced as more carrier accumulate at the tumor site which will increase the efficacy of the treatment[11][12]. Besides that, by introducing the targeting agent, drug delivery is not completely dependent on the EPR effect, and this active targeting can reach hematological malignancies, small metastatic tumors and others that do not exhibit the EPR effect[13] . In order to achieve a good targeting strategy, the ligand density is the key factor. In the case of high-density of targeting ligand, it will cause other effects such as increased the clearance possibility, increased of particles size which will minimize the EPR effect, a steric hindrance which will reduce the binding capability and reduced cellular uptake[14]. The common targeting ligands functionalized on MSNs are summarized in Figure 1.
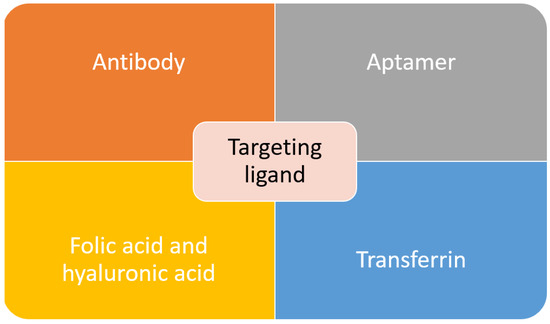
Figure 1. Common targeting ligands functionalized on MSNs.
Antibodies, also known as an immunoglobulin (Ig), are one of the important factors to develop an active targeting drug delivery agent. Through the identification of an antigen specifically overexpressed on cancer cells, complement antibodies can be used to lead the carrier to the tumor site. Antibody targeting agent posed a few advantages over other compounds due to its specificity and selectivity. It typically exhibited “Y” shape protein, which consists of two heavy and two light chains. The two arms served as recognition sites for antigen, while the stem serves as effector functions. There are several factors that will affect the efficacy of a targeted system, such as antibodies’ configuration, origin and mode of linkage to the carrier [14][15][16]. There have been many reports on the functionalization of the antibody as a targeting agent to the MSNs. One work report on targeting prostate cancer through MSNs conjugation with prostate-specific membrane antigen (PSA) antibody. The PSA antibody is attached to the MSNs through the esterification reaction between the carboxyl group and an amino group . In another work, to target retinablastoma (RB), an antibody was conjugated with MSNs to deliver carboplatin. It was found that in RB, there is overexpression of epithelial cell adhesion molecule (EpCAM), and its inhibition will result in poor cell proliferation. Therefore, this work reported on the conjugation of EpCAM antibody on MSNs through esterification with the carboxyl group on MSNs surface[16]. To target HeLa cells, a monoclonal anti-human epidermal growth factor receptor (EGFR) antibody, clone AT6E3 was conjugated to MSNs composite. The antibody was attached to the graphene oxide (GO) surface of MSNs composite. The result showed that with antibody conjugation, the carrier was internalized and retained in HeLa cells, and this indicated the effectiveness of the antibody in cancel targeting and treatment[17]. The FDA-approved antibody trastuzumab, which targets the HER2 receptor, was conjugated with MSNs to target breast cancer cells[18][19][20]. Although antibodies are effective in targeting the desired cancer cell, their high cost and potential adverse immune response have caused the research of another alternative targeting agent.
Another class of compound that can be used as a targeting agent are aptamers. They are defined as short RNA or single-stranded DNA oligonucleotides or oligopeptides (5–80 amino acids), which can bind to specific targets through folding into unique three-dimensional conformations[21][22]. Aptamers have unique characteristics such as small size, high stability, structure flexibility, ease of synthesis, and low or no immunogenicity, and these make them a great targeting agent [23]. In recent work, MSNs were successfully functionalized with AS1411 DNA aptamer as a targeting agent towards colorectal adenocarcinoma. This aptamer was bonded to the PEG that covering the MSNs surface [24]. DNA aptamer specifically targeting mucin-1 (MUC-1), was successfully functionalized to the MSNs. MUC-1 is highly expressed on the surface of breast cancer, ovarian and lung cancer and in this study, the efficacy of the carrier was determined against MCF-7 human breast cancer cell [25]. Tuna and coworkers reported on the usage of aptamer as both the capping and targeting agent of MSNs carrier. The aptamer, nucleolin AS1411, was used to close the MSNs pores to entrap the cargo, carbendazim. This carrier was tested against human cervical adenocarcinoma cells. The results showed that MSNs with aptamer gate exhibited a 2.3-fold increase of toxicity to the target cells compare to pure carbendazim[26].
Through our literature search, we discovered that the most commonly used targeting agent is folic acid. It is also commonly known as vitamin B9, and it can bind with folate receptors. There are three isoforms of folate receptors, namely hFRα, hFRβ and hFRγ. hFRα is the most commonly targeted as it is overexpressed across various cancers such as uterus, ovary, breast, cervix, kidney, colon and testicular. Various works have reported on the functionalization of folic acid on MSNs surface to target breast cancer [27][28][29][, lung cancer[30][31], leukemia[32] and pancreatic cancer[33]. Although folic acid is an effective targeting agent, the presence of folate receptors on normal cells can interfere with the efficacy of the drug delivery agent. Another receptor that is overexpressed in solid tumors such as pancreas, breast and lung cancer is a cluster of differentiation-44 (CD-44). The commonly used targeting agent for this receptor is hyaluronic acid. It is an anionic nonsulfated glycosaminoglycan component of the extracellular matrix. It is also biocompatible and nonimmunogenic. Thus, it is frequently used in biomedical applications. There have been several works that report on the functionalization of hyaluronic acid on MSNs as a targeting agent[34][35][36][37]. Interestingly, one study reported on the use of hyaluronic acid as both pore closing of MSNs and targeting agent of the carrier. To close the MSNs’ pores, a pH-sensitive group was bonded between the MSNs and hyaluronic acid. The carrier will reach the target site using hyaluronic acid as the ligand, and at the tumor site where the pH is lower, the bond connecting hyaluronic acid with MSNs will be broken, and the drug will be released [38].
Transferrin is another type of ligand that can be used to target cancer cells with overexpression of transferrin receptors. Transferrin can facilitate the nanoparticle entrance to the cancer cells through receptor-mediated endocytosis pathway. Pallares and colleagues reported on the delivery of MSNs carrier using transferrin as targeting ligand towards breast cancer[39]. A derivative of transferrin, ferritin, also has been used as the targeting agent to target the transferrin receptor. In this work, ferritin was used as both the gate for the MSNs pores and targeting agent [40]. Pancreatic cancer cell was successfully targeted using transferrin conjugated polymer-coated MSNs with gemcitabine as the anticancer drug[41]. There were many other works that utilized transferrin as a ligand, but it will not be covered in this review. The final popular targeting ligand is arginine-glycine-aspartic acid (RGD) peptide, which can target the integrin receptor. Integrin receptors are found to be overexpressed on angiogenetic endothelial cells and certain tumors, but they are absent in normal cells. This makes them an attractive receptor to be targeted in cancer therapy using RGD peptide[11]. Recently, Yan and coworkers reported on dual ligand using RGD peptide and folic acid functionalized MSNs to target human breast cancer MCF-7 cell[42]. Similar to previous reports, peptide-containing RGD motif was used as both the gate to cover the MSNs pores and targeting agent[43]. Overall, there are various targeting agents that can be used to improve the properties of MSNs as drug delivery agents. Although active targeting is able to improve the efficacy of anticancer drug delivery, the risk of premature release is still possible. Therefore, the research to discovering an improved carrier has evolved towards a stimulus-responsive system, which will be further discussed in the upcoming section.
2.3. Stimulus Responsive
The development of MSNs as drug delivery agents evolved from passive targeting, active targeting and now stimulus responsive system. Based on our literature search, the general form of a stimulus-responsive system utilizes both passive targeting and active targeting simultaneously with an additional gate to trap the cargo. The weakness of both passive and active targeting is premature to release of cargo during circulation; therefore, a gate was employed to close the pores of MSNs. This gate will be open once it reaches the target sites through a specific stimulus (Figure 3). The type of stimulus can be classified into two categories: internal and external stimulus. However, in this review, we will be categorizing the responsive stimulus system into the number of stimuli, which are single stimuli and multiple stimuli.
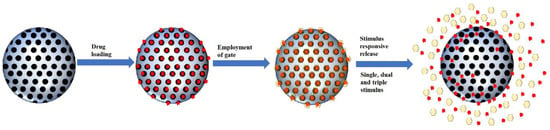
Figure 3. Schematic illustration of smart MSNs with the stimulus-responsive system.
2.3.1. Single Stimulus
As was mention previously, there are two types of stimuli, which are internal, such as pH, redox and enzyme, and external stimuli, such as light and temperature. In this section, we will be covering the common stimulus for both types of stimuli with the focus on the single-stimulus-responsive-MSNs system. Table 2 summarizes the research that utilized single-stimulus-responsive-MSNs for delivery of the anticancer drug. The gatekeeper and stimuli type are also indicated.
|
Gatekeeper |
Stimulus |
Cargo/Drugs |
Ref |
|
Polyacrylic acid |
pH |
Umbelliferone |
[28] |
|
Poly-L-lysine |
pH |
Doxorubicin |
[44] |
|
Poly(tannic acid) |
pH |
Doxorubicin |
[45] |
|
Hyaluronic acid |
pH |
Doxorubicin |
[35] |
|
Polyethylene glycol and chitosan |
pH |
Doxorubicin |
[46] |
|
Zinc oxide |
pH |
Doxorubicin |
[47] |
|
Albumin |
pH |
Lamivudine |
[47] [47] |
|
Ferritin |
pH |
Doxorubicin |
[40] |
|
Beta-cyclodextrin |
pH |
5-fluorouracil |
[48] |
|
Chitosan |
Redox |
Rhodamine 6G |
[49] |
|
Cucurbit[6]uril, cyclopentyl methylamine and polyacrylic acid |
Redox |
Doxorubicin |
[50] |
|
Gold |
Redox |
Doxorubicin |
[51] |
|
Zinc sulphide |
Redox |
Doxorubicin |
[52] |
|
Ferrocene-containing amphiphilic block copolymer |
Redox |
Doxorubicin |
[53] |
|
Organosilica |
Redox |
Doxorubicin |
[54] |
|
Graphene |
Redox |
Rhodamine B |
[55] |
|
Bovine serum albumin |
Redox |
Epirubicin |
[56] |
|
Chitosan |
Enzyme |
Doxorubicin |
[57] |
|
Peptide |
Enzyme |
Organotin |
[58] |
|
Guar gum |
Enzyme |
5-fluorouracil |
[59] |
|
Hyaluronic acid |
Enzyme |
5-fluorouracil |
[60] |
|
Hyaluronic acid |
Enzyme |
Doxorubicin |
[61] |
|
Hyaluronic acid and collagen I |
Enzyme |
Doxorubicin |
[62] |
|
Iron oxide |
Enzyme |
Doxorubicin |
[63] |
|
Gold |
Enzyme |
Doxorubicin |
[64] |
|
Ruthenium complex |
Light |
Sarafanin O |
[65] |
|
α-cyclodextrin |
Light |
Doxorubicin |
[66] |
|
β-cyclodextrin |
Light |
Doxorubicin |
[67] |
|
β-cyclodextrin |
Light |
Camptothecin |
[68] |
|
β-cyclodextrin and -diazo-1.2-napthoquinones |
Light |
Doxorubicin |
[69] |
|
Human serum albumin |
Light |
Doxorubicin |
[70] |
|
Supported lipid bilayer |
Temperature |
Doxorubicin |
[71] |
|
Poly(N-isopropylacrylamine)-co-poly(methacrylic acid) (PNINAM-co-PMAA) |
Temperature |
Doxorubicin |
[72] |
|
Poly(N-isopropylacrylamide)-co-(1-butyl-3-vinyl imidazolium bromide) (p-NIBIm) |
Temperature |
Cytochrome C |
[73] |
|
PNINAM |
Temperature |
Methylene Blue |
[74] |
pH-responsive MSNs is one of the promising vehicles to deliver anticancer drugs to the targeted region. This system was developed through the exploitation of pH values in most tumors, which is lower compared to normal cells. The lower pH (around 6.5) value is due to the Warburg effect, where the cancer cells produce energy through glycolysis with or without oxygen, and this leads to the production of acidic lactate[75] [197]. Furthermore, after the internalization of the carrier to the cancer cells, it will be exposed to even more acidic media in endosomal (pH 5.5–6.0) and lysosomal (pH 4.5–5.0). Kundu and colleagues reported on the delivery of umbelliferone, a plant-derived natural product, using pH-sensitive MSNs. The pores of MSNs were capped with polyacrylic acid, pH-sensitive polymer, and the surface was grafted with folic acid as the targeting agent. The drug was released under acidic pH due to the less electrostatic interaction and dissociation of the amide bond between the MSNs and the polymer[28]. In another work, poly-l-lysine gated MSNs were used to deliver doxorubicin. In a neutral environment, poly-l-lysine shrunk, which formed a dense barrier on the pore entrance. As the pH reduced, the polymer is swollen, and the drug was released[44] One work reported on the usage of zinc oxide quantum dots as the gate to trap doxorubicin in the MSNs pores. The drug released at lower pH as the zinc oxide quantum dots dissolve in acidic condition and this lead to drug released[47]. Chen and coworkers successfully produced pH-responsive MSNs with “release-stop-release” properties. Typical pH-responsive MSNs will be activated in acidic conditions, and the process is not reversible. In this work, they managed to produce pH-responsive MSNs with reversible function. They utilized poly(tannic acid) as the gatekeeper with tetrethylenepentamine (TEPA) as a crosslinker. In acidic conditions, TEPA was protonated, thus increasing its hydrophilicity. This resulted in poly(tannic acid) swelling and substantial drug release. As the pH increased, the TEPA deprotonated and poly(tannic acid) will become dense again, thus closed the pores of MSNs. The authors stated that through this system, the side effect of the leftover drug could be minimized as safely excreted out[76].
Utilizing redox potential is another pathway to develop a stimulus responsive drug delivery agent. In a cancerous cell, the production of reactive oxygen species (ROS) is elevated due to genetic mutation, mitochondrial dysfunction and different metabolism. To overcome this, the production of ROS scavenger, the prominent being tripeptide glutathione (GSH), is also increased. It should be noted that GSH also presents in normal cells, but in cancerous cells, it contributes to the cancer progression and responsible for the resistance increment of radio- and chemoresistance of cancer cells[14]. It was found that the concentration of GSH is significantly higher in cancer cells (approximately 2–20 mM) compared to the extracellular part (2–20 nM)[77]. By utilizing the difference in the GSH concentration, the release of drugs from redox responsive MSNs upon cancer cell entrance will occur. One work reported on the utilization of redox potential to produce a redox responsive system with chitosan as the gate to trap the drug in the MSNs pore. Interestingly, this work used a fluorescent agent as the anchoring molecule which binds the chitosan with MSNs. This fluorescent agent was reported to be sensitive to GSH. Once the carrier entered the cancer cell, this fluorescence agent will be cleaved, and chitosan will be released from the carrier. Therefore, the drug will be released to treat the cancerous cell [49]. Thiol-linker is one of the most utilize linker to develop redox responsive MSNs. This linker will be broken by GSH, which will lead to the drug release. Zhang and coworkers reported on the redox responsive system with gold (Au) nanoparticles as the gate to the MSNs. The Au was bonded to the MSNs surface using a thiol-linker and exposure of this carrier to high GSH concentration’s environment, the linker was broken, and the drug, doxorubicin, was released[78]. In another work, the redox responsive MSNs was developed through the layer-by-layer assembly of curcubit-6-uril and cyclopentyl methylamine polymer, which served as the gate to cover the MSNs pores. This work studied the effect of layers’ numbers on the drug release activity with redox stimulus. In the presence of GSH, the percentage of drug released from the carrier decreased with increased layer number. They concluded that the cargo release rate could be manipulated through the variation of polymer layers[79].
Another stimulus-responsive system that is popular is the enzyme responsive MSNs carrier. Utilization of enzyme as stimulus poses several advantages such as high chemical selectivity, substrate specificity and mild condition. For cancer treatment, there is a specific enzyme present in this tumor microenvironment. Therefore, by employing a carrier with a component that sensitive to the present enzyme, a control released system through enzymatic cleavage is obtained. Cai et al. reported on the production of enzyme responsive MSNs with chitosan as the gate responsible for trapping the drug in the MSNs pores. The component that was sensitive to the enzyme is the azo bond linked to the MSNs and chitosan. The results showed that the azo bond was broken in the presence of colon enzyme secreted by colonic microflora[57]. In another work, the same enzyme was used as a response to MSNs with guar gum as the pores’ cap. The colon enzyme degraded the guar gum, and the drug, 5-fluorouracil, was released[59]. Functionalization of MSNs with hyaluronic acid was found to be an enzyme responsive carrier in addition to active targeting[61]. Hyaluronic acid as a targeting agent can bind with CD-44 receptor, which is found in most cancer cells, and degraded by the enzyme hyaluronidase. One work used hyaluronic acid as both the gate and targeting agent [60]. Zhou and colleagues reported on the production of MSNs conjugated with hyaluronic acid and collagen I matrix for enzyme responsive system. Interestingly, this carrier was reported to be responsive to two enzymes, hyaluronidase and metalloproteinase-2. Through drug released studies, it was found that in the absence of enzymes, no drug was detected, while in the presence of an enzyme, up to 75% of the drug was released[62].
Other than internal stimulus, as stated previously, an external stimulus such as light and temperature were found to be commonly applied in the development of a responsive delivery system using MSNs. To generate light responsive MSNs, functionalization using a photoactive group is a need. The trigger for drug release will be light with various wavelengths such as ultraviolet (UV), visible (Vis) and near-infrared (NIR) [13]. Wang and coworkers reported on UV responsive MSNs carrier for anticancer treatment. The pore of MSNs was capped with α-cyclodextrin with hydrazone bond linking the azobenzene functionalized MSNs and α-cyclodextrin. Through UV irradiation, the azobenzene conformation changed, which led to the dissociation of α-cyclodextrin and the drug, doxorubicin, released[67]. Although this work showed promising results, UV light as a trigger is not practical as it poses an adverse effect on the cell and tissue and has low tissue penetrability[80]. Visible light has also been reported as a trigger to release the cargo from MSNs. One work reported on the usage of the ruthenium complex as the gate to cap the MSNs’ pores. The result indicated that in the absence of light, the carrier was stable, and when Vis light was irradiated, the rapidly released cargo was observed. Interestingly, the gate, the ruthenium complex, can be controlled. Through heating at elevated temperatures, the pores re-closed, which can be re-opened upon irradiation[65]. Visible light can overcome the problem posed by UV, but it still has poor tissue penetration, which makes it difficult to be applied in cancer treatment. The best light wavelength to achieve good tissue penetration lies between 650–900 nm, which is in the NIR range. Therefore, the majority of light-responsive MSNs developed with NIR trigger[80]. One work reported on Janus gold nanostar-MSNs as NIR responsive carrier. The gold surface was functionalized with a thiolated photolabile molecule to bind itself to MSNs and β-cyclodextrin as the gate. Through NIR irradiation, the photolabile molecule dissociated and form succinic acid and this succinic acid induced the opening of the gate. In another work, upconversion nanoparticles (UCNP) were coated with MSNs layer, and the gate employed was β-cyclodextrin. The β-cyclodextrin was linked to the MSNs layer with photo-cleavable pyrenemethyl ester. The principle of this light-responsive system as follows: 1) NIR light was irradiated to the carrier. Due to the presence of UCNP, the NIR was converted to Vis and UV light. 2) The pyrenemethyl ester bond was broken with higher energy light, and the β-cyclodextrin was dissociated, which led to the drug released[68].
Temperature is another stimulus being used to develop stimulus responsive MSNs. The important criteria in producing thermo-responsive MSNs are it should be stable during the circulation (37 °C) and the drug released at the locally heated tumor (~40–45 °C)[81]. One work reported on the production of thermo-responsive MSNs by incorporating a supported lipid bilayer (SBL) on the MSNs surface. This SBL also served as the gate to close the MSNs pores and trap the drug. At elevated temperature, the lipid bilayer became flexible and exhibited a liquid state. This caused it to be more permeable, which led to the drug released[71] [193]. Besides that, the thermo-responsive polymer was used to coat the MSNs surface to develop thermo-responsive MSNs. Copolymer consisting of poly(N-isopropylacrylamine) and poly(methacrylic acid) (PNINAM-co-PMAA) was used as the coating of MSNs surface to trap the drug, doxorubicin. This copolymer served as both the gate and thermo-responsive component of the carrier. At 37 °C, which is lower than the low critical solution temperature (LCST), the copolymer swelled and blocked the MSNs pores. As the temperature increased (>LCST), the copolymer collapsed, and the drug was released [72]. Overall, through our literature search, we discovered that the articles on MSNs carriers that depend solely on temperature stimulus are lacking. Most articles report on the production of dual or more stimulus-responsive MSNs, which consists of temperature and any other stimulus.
2.2.2. Multiple Stimulus
In recent years, we discovered that the production of stimulus-responsive-MSNs as a drug delivery agent is no longer limited to single stimuli. Most articles reported on the utilization of dual-responsive systems, and some even reported up to three responsive systems. The most common stimulus being used simultaneously is pH and redox stimulus. Lu and colleague reported on the usage of hollow MONs with disulfide bridge in the silica walls and supramolecular interaction between α-cyclodextrin and anilino alkane group as the gate to produce pH and redox responsive carrier. The results showed that at low pH (mimicking the tumor environment), the percentage of doxorubicin released was about 58%. This was reported due to the dissociation of α-cyclodextrin from protonated aniline. As state previously, the disulfide bond is susceptible to GSH, which present at a high concentration in cancerous cells. GSH will cleave the disulfide bond resulted in the breaking of hollow MONs walls, and the drug was released. Through our literature search, there were three articles reported on the utilization of chitosan and disulfide bond to create pH and redox responsive MSNs. Chitosan is a pH-responsive biopolymer. In acidic conditions, chitosan may disassemble and lead to the pore opening and drug being released. By using a disulfide bond to link the MSNs and chitosan, a redox responsive system was created. With a high concentration of GSH, the disulfide bond was broken, which lead to chitosan gate opening and release of the drug[82][83][84]. Besides simultaneous use of pH and redox stimulus, there have been reports on pH and ROS stimulus being used. Interestingly, Song et al. reported that polydopamine served as both the pH and ROS responsive component of the carrier. At low pH, the amine group of polydopamine was protonated, which broke the interaction between the polymer and curcumin (drug). In the presence of hydrogen peroxide (ROS stimulus), the hydrogen bond between polydopamine and curcumin was broken, and the drug was released [85]. Dual responsive MSNs that are sensitive towards pH and temperature has also been developed. MSNs consist of carbon dots and poly(N-vinylcaprolactam) was produced as a pH and temperature-responsive carrier. The carbon dots and poly(N-vinylcaprolactam) were functionalized to the MSNs surfaces using a Schiff base bond. This Schiff base was reported to be acid sensitive and broken at acid atmosphere, which led to drug released. The polymer, poly(N-vinylcaprolactam), which served as a temperature-responsive molecule were at an elevated temperature; the polymer underwent phase transition and collapsed where the drug was then released from the carrier[86]. There have been other dual responsive systems reported and are summarized in Table 2.
Table 2. Multiple responsive MSNs with their responsive linker/moiety.
|
Responsive linker/moiety |
Stimulus |
Ref |
|||||||
|
pH |
Redox |
Enzyme |
ROS |
Temperature |
Light |
Other compounds |
|
||
|
Dual stimulus |
|||||||||
|
α-cyclodextrin and anilinoalkane |
Disulfide bond |
|
|
|
|
|
|
||
|
Polydopamine |
Disulfide bond |
|
|
|
|
|
[87] |
||
|
Chitosan |
Disulfide bond |
|
|
|
|
|
[88] | ||
|
Citraconic |
Disulfide bond |
|
|
|
|
|
[89] |
||
|
Chitosan |
Disulfide bond |
|
|
|
|
|
|||
|
Benzoic imine bonds |
Disulfide bond |
|
|
|
|
|
[36] |
||
|
Zinc oxide quantum dots |
Disulfide bond |
|
|
|
|
|
[90] |
||
|
Bull serum albumin |
Disulfide bond |
|
|
|
|
|
[91] |
||
|
Sodium alginate |
Disulfide bond |
|
|
|
|
|
[92] |
||
|
Polydopamine |
|
|
Polydopamine |
|
|
|
[85] |
||
|
Carboxymethyl chitin |
|
|
Thioketal bond |
|
|
|
[93] |
||
|
Schiff base bonds |
|
|
|
Poly(N-vinylcaprolactam) |
|
|
[86] |
||
|
PEG-like Jeffamine M-2005 |
|
|
|
Polyphosphazene |
|
|
[94] |
||
|
Poly(N-isopropylacrylamide-co-methacrylic acid) |
|
|
|
Poly(N-isopropylacrylamide-co-methacrylic acid) |
|
|
[95] |
||
|
Polydopamine |
|
|
|
|
Gold |
|
[17] |
||
|
Hydroxyapatite |
|
|
|
|
Gold |
|
[96] |
||
|
Carboxylic acid |
|
|
|
|
|
Chloride ions (Salt) |
[97] |
||
|
|
Disulfide bond |
Cystine-dopamine |
|
|
|
|
[98] |
||
|
|
Dithiodipropionic |
|
Selenocystine |
|
|
|
[99] |
||
|
|
Disulfide bond |
|
|
Poly(γ-benzyl-l-glutamate) |
|
|
[100] |
||
|
|
Disulfide bond |
|
|
|
Azobenzene / Galactose-grafted polymer |
|
[101] |
||
|
|
|
|
Ferrocene |
Poly(N-isopropylacrylamide) |
|
|
[102] |
||
|
Triple stimulus |
|||||||||
|
Amide bond |
Gold-sulphur bond |
Hyaluronic acid |
|
|
|
|
[103] |
||
|
Schiff base bond |
Disulfide bond |
|
|
poly(N-isopropylacrylamide-block-poly(2-(4-formylbenzoyloxy) ethyl methacrylate) |
|
|
[104]
|
||
|
Polydopamine |
Disulfide bond |
|
|
|
Polydopamine |
|
[105] |
||
|
Electrostatic interaction |
Disulfide bond |
|
|
|
Carbon dots |
|
[105] |
||
|
Ester bond |
Disulfide bond |
|
|
|
|
Molecular interaction (Glucose) |
[106] |
||
Besides dual responsive systems, there have been reports on tiple responsive systems. pH and redox the common stimuli being used while the third stimulus varied. Chen and coworkers reported on triple responsive carriers, which were sensitive to pH, redox and enzyme stimuli. The MSNs were functionalized with gold via a sulfide bond and hyaluronic acid via an amide bond. The gold–sulfur bond, amide bond and hyaluronic acid were reported to be responsive to the redox, pH and enzyme stimulus, respectively[103]. In other work, a triply responsive system was developed to be sensitive towards pH, redox and temperature. The carrier consisted of multiwalled carbon nanotubes covered with mesoporous silica graft poly(N-isopropylacrylamide-block-poly(2-(4-formylbenzoyloxy) ethyl methacrylate) via disulfide linkages. As stated previously, the disulfide link is receptive towards redox stimulus. The drug was bonded to the polymer via Schiff base bond, and this bond was broken in acidic condition, which led to doxorubicin released. At elevated temperatures, the polymer collapsed. Thus, the drug was released[107]. Other articles related to triple responsive MSNs are summarized in Table 2.
2.4. Multidrug Carrier
Chemotherapy is a systemic treatment that poses a therapeutic effect on tumors with a tendency to metastasized to advanced tumors. However, there are several issues that need to be overcome, such as (1) Adverse side effects, (2) Most chemotherapeutic drugs have low aqueous solubility poor stability, (3) Poor delivery to achieve good efficacy and (4) Multidrug resistance (MDR). In a typical chemotherapy process, multiple drugs administered sequentially to increase treatment efficacy and delay[108]. An alternative to overcome these issues is by incorporating two or more drugs in a single carrier. They have been several reports that utilized MSNs as a carrier to deliver two anticancer drugs for treatment. Zhang and colleagues reported on the usage of hollow MONs to deliver doxorubicin and cisplatin drugs. The results showed the with two drugs; the anticancer activity was enhanced[109]. For the treatment of acute promyelocytic leukemia, MSNs were coated with folic acid-modified PEGylated lipid bilayer membrane to carrier paclitaxel and tanshinone IIA drugs[32]. Specifically, to overcome the MDR of cancer stem cells, MSNs were used to deliver doxorubicin and tariquidar[110]. MSNs carrier consists of polydopamine, and upconversion nanoparticles in yolk-shell configuration were produced to deliver doxorubicin and hydroxycamptothecin. This carrier was able to deliver both hydrophilic (doxorubicin) and hydrophobic (hydroxycamptothecin), where MSNs were used for doxorubicin and polydopamine for hydroxycamptothecin housing, respectively[111]. Similarly, hydrophilic and hydrophobic drug, pemetrexed and ellagic acid, respectively, were loaded onto MSNs. The ellagic acid was encapsulated within the MSNs pores through electrostatic interaction while pemetrexed was chemically anchored to the lactoferrin shell which surrounds the MSNs [112]. Interestingly, Janus nanoparticles consist of gold and MSN with an additional β-cyclodextrin gate was successfully synthesized to deliver paclitaxel and doxorubicin. The β-cyclodextrin served as the carrier for paclitaxel, while MSNs cargo was doxorubicin[113]. Overall, MSNs application as a drug delivery agent is not limited to anticancer or chemotherapeutic drugs; it can also serve as a carrier for gene therapy, immunotherapy or photothermal therapy.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13020152
References
- Pednekar, P.P.; Godiyal, S.C.; Jadhav, K.R.; Kadam, V.J. Chapter 23—Mesoporous silica nanoparticles: A promising multi-functional drug delivery system. In Nanostructures for Cancer Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amster-dam, The Netherlands, 2017; pp. 593–621. doi:10.1016/B978-0-323-46144-3.00023-4.
- Kresge, C.T.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E. The discovery of ExxonMobil's M41S family of mesoporous molecu-lar sieves. In Studies in Surface Science and Catalysis; Terasaki, O., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 148, pp. 53–72.
- Habeche, F.; Hachemaoui, M.; Mokhtar, A.; Chikh, K.; Benali, F.; Mekki, A.; Zaoui, F.; Cherifi, Z.; Boukoussa, B. Recent Ad-vances on the Preparation and Catalytic Applications of Metal Complexes Supported-Mesoporous Silica MCM-41 (Review). J. Inorg. Organomet. Polym. Mater. 2020, doi:10.1007/s10904-020-01689-1.
- Rahikkala, A.; Pereira, S.A.P.; Figueiredo, P.; Passos, M.L.C.; Araújo, A.R.T.S.; Saraiva, M.L.M.F.S.; Santos, H.A. Mesoporous Silica Nanoparticles for Targeted and Stimuli-Responsive Delivery of Chemotherapeutics: A Review. Adv. Biosyst. 2018, 2, doi:10.1002/adbi.201800020.
- Chew, T.L.; Ahmad, A.L.; Bhatia, S. Ordered mesoporous silica (OMS) as an adsorbent and membrane for separation of car-bon dioxide (CO2). Adv. Colloid Interface Sci. 2010, 153, 43–57, doi:10.1016/j.cis.2009.12.001.
- Narum, S.M.; Le, T.; Le, D.P.; Lee, J.C.; Donahue, N.D.; Yang, W.; Wilhelm, S. Chapter 4-Passive targeting in nanomedicine: Fundamental concepts, body interactions, and clinical potential. In Nanoparticles for Biomedical Applications; Chung, E.J., Leon, L., Rinaldi, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 37–53. 10.1016/B978-0-12-816662-8.00004-7.
- Martinez-Carmona, M.; Colilla, M.; Vallet-Regi, M. Smart Mesoporous Nanomaterials for Antitumor Therapy. Nanomaterials 2015, 5, 1906–1937, doi:10.3390/nano5041906.
- Ku, S.; Yan, F.; Wang, Y.; Sun, Y.; Yang, N.; Ye, L. The blood-brain barrier penetration and distribution of PEGylated fluo-rescein-doped magnetic silica nanoparticles in rat brain. Biochem. Biophys. Res. Commun. 2010, 394, 871–876, doi:10.1016/j.bbrc.2010.03.006.
- Watermann, A.; Brieger, J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials 2017, 7, doi:10.3390/nano7070189.
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and can-cer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924, doi:10.7150/thno.49577.
- Barui, S.; Cauda, V. Multimodal Decorations of Mesoporous Silica Nanoparticles for Improved Cancer Therapy. Pharmaceu-tics 2020, 12, 33, doi:10.3390/pharmaceutics12060527.
- Behera, A.; Padhi, S. Passive and active targeting strategies for the delivery of the camptothecin anticancer drug: A review. Environ. Chem. Lett. 2020, 18, 1557–1567, doi:10.1007/s10311-020-01022-9.
- Tarudji, A.W.; Kievit, F.M. Chapter 3-Active targeting and transport. In Nanoparticles for Biomedical Applications; Chung, E.J., Leon, L., Rinaldi, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 19–36. doi:10.1016/B978-0-12-816662-8.00003-5.
- Gisbert-Garzaran, M.; Vallet-Regi, M. Influence of the Surface Functionalization on the Fate and Performance of Mesopo-rous Silica Nanoparticles. Nanomaterials 2020, 10, doi:10.3390/nano10050916.
- Salahpour Anarjan, F. Active targeting drug delivery nanocarriers: Ligands. Nano-Struct. Nano-Objects 2019, 19, 100370, doi:10.1016/j.nanoso.2019.100370.
- Attarwala, H. Role of antibodies in cancer targeting. J. Nat. Sci. Biol. Med. 2010, 1, 53–56, doi:10.4103/0976-9668.71675.
- Tran, V.A.; Vo, V.; Shim, K.; Lee, S.W.; An, S.S.A. Multimodal Mesoporous Silica Nanocarriers for Dual Stimuli-Responsive Drug Release and Excellent Photothermal Ablation of Cancer Cells. Int. J. Nanomed. 2020, 15, 7667–7685, doi:10.2147/ijn.S254344.
- Yamaguchi, H.; Hayama, K.; Sasagawa, I.; Okada, Y.; Kawase, T.; Tsubokawa, N.; Tsuchimochi, M. HER2-Targeted Multi-functional Silica Nanoparticles Specifically Enhance the Radiosensitivity of HER2-Overexpressing Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, doi:10.3390/ijms19030908.
- Ngamcherdtrakul, W.; Sangvanich, T.; Reda, M.; Gu, S.; Bejan, D.; Yantasee, W. Lyophilization and stability of anti-body-conjugated mesoporous silica nanoparticle with cationic polymer and PEG for siRNA delivery. Int. J. Nanomed. 2018, 13, 4015–4027, doi:10.2147/ijn.S164393.
- Li, L.; Lu, Y.; Jiang, C.; Zhu, Y.; Yang, X.; Hu, X.; Lin, Z.; Zhang, Y.; Peng, M.; Xia, H.; et al. Actively Targeted Deep Tissue Imaging and Photothermal-Chemo Therapy of Breast Cancer by Antibody-Functionalized Drug-Loaded X-Ray-Responsive Bismuth Sulfide@Mesoporous Silica Core–Shell Nanoparticles. Adv. Funct. Mater. 2018, 28, 1704623, doi:10.1002/adfm.201704623.
- Guan, B.Z.; Zhang, X.W. Aptamers as Versatile Ligands for Biomedical and Pharmaceutical Applications. Int. J. Nanomed. 2020, 15, 1059–1071, doi:10.2147/ijn.S237544.
- Sosnik, A. Chapter 1-From the “Magic Bullet” to Advanced Nanomaterials for Active Targeting in Diagnostics and Thera-peutics. In Biomedical Applications of Functionalized Nanomaterials; Sarmento, B., das Neves, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–32. doi:10.1016/B978-0-323-50878-0.00001-X.
- Vandghanooni, S.; Barar, J.; Eskandani, M.; Omidi, Y. Aptamer-conjugated mesoporous silica nanoparticles for simultaneous imaging and therapy of cancer. Tractrends Anal. Chem. 2020, 123, doi:10.1016/j.trac.2019.115759.
- Babaei, M.; Abnous, K.; Taghdisi, S.M.; Taghavi, S.; Sh. Saljooghi, A.; Ramezani, M.; Alibolandi, M. Targeted rod-shaped mesoporous silica nanoparticles for the co-delivery of camptothecin and survivin shRNA in to colon adenocarcinoma in vitro and in vivo. Eur. J. Pharm. Biopharm. 2020, 156, 84–96, doi:10.1016/j.ejpb.2020.08.026.
- Si, P.; Shi, J.; Zhang, P.; Wang, C.; Chen, H.; Mi, X.; Chu, W.; Zhai, B.; Li, W. MUC-1 recognition-based activated drug nano-platform improves doxorubicin chemotherapy in breast cancer. Cancer Lett. 2020, 472, 165–174, doi:10.1016/j.canlet.2019.12.019.
- Tuna, B.G.; Atalay, P.B.; Kuku, G.; Acar, E.E.; Kara, H.K.; Yilmaz, M.D.; Ozalp, V.C. Enhanced antitumor activity of car-bendazim on HeLa cervical cancer cells by aptamer mediated controlled release. Rsc Adv. 2019, 9, 36005–36010, doi:10.1039/c9ra07974b.
- Li, Y.; Wang, S.; Song, F.X.; Zhang, L.; Yang, W.; Wang, H.X.; Chen, Q.L. A pH-sensitive drug delivery system based on folic acid-targeted HBP-modified mesoporous silica nanoparticles for cancer therapy. Colloids Surf. Physicochem. Eng. Asp. 2020, 590, 124470, doi:10.1016/j.colsurfa.2020.124470.
- Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P.C. Tumor targeted delivery of umbelliferone via a smart mesoporous silica nanoparticles controlled-release drug delivery system for increased anticancer efficiency. Mater. Sci. Eng. C 2020, 116, 111239, doi:10.1016/j.msec.2020.111239.
- Sheena, T.S.; Dhivya, R.; Rajiu, V.; Jeganathan, K.; Palaniandavar, M.; Mathan, G.; Akbarsha, M.A. Folate-engineered meso-porous silica-encapsulated copper (II) complex [Cu(L)(dppz)]+: An active targeting cell-specific platform for breast cancer therapy. Inorg. Chim. Acta 2020, 510, 119783, doi:10.1016/j.ica.2020.119783.
- Song, Y.; Zhou, B.; Du, X.; Wang, Y.; Zhang, J.; Ai, Y.; Xia, Z.; Zhao, G. Folic acid (FA)-conjugated mesoporous silica nano-particles combined with MRP-1 siRNA improves the suppressive effects of myricetin on non-small cell lung cancer (NSCLC). Biomed. Pharmacother. 2020, 125, 109561, doi:10.1016/j.biopha.2019.109561.
- Malekmohammadi, S.; Hadadzadeh, H.; Amirghofran, Z. Preparation of folic acid-conjugated dendritic mesoporous silica nanoparticles for pH-controlled release and targeted delivery of a cyclometallated gold(III) complex as an antitumor agent. J. Mol. Liq. 2018, 265, 797–806, doi:10.1016/j.molliq.2018.07.024.
- Li, Z.; Zhang, Y.; Zhu, C.; Guo, T.; Xia, Q.; Hou, X.; Liu, W.; Feng, N. Folic acid modified lipid-bilayer coated mesoporous silica nanoparticles co-loading paclitaxel and tanshinone IIA for the treatment of acute promyelocytic leukemia. Int. J. Pharm. 2020, 586, 119576, doi:10.1016/j.ijpharm.2020.119576.
- Li, H.; Li, K.; Dai, Y.; Xu, X.; Cao, X.; Zeng, Q.; He, H.; Pang, L.; Liang, J.; Chen, X.; et al. In vivo near infrared fluorescence imaging and dynamic quantification of pancreatic metastatic tumors using folic acid conjugated biodegradable mesoporous silica nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1867–1877, doi:10.1016/j.nano.2018.04.018.
- Ghosh, S.; Dutta, S.; Sarkar, A.; Kundu, M.; Sil, P.C. Targeted delivery of curcumin in breast cancer cells via hyaluronic acid modified mesoporous silica nanoparticle to enhance anticancer efficiency. Colloids Surf. B. Biointerfaces 2021, 197, 111404, doi:10.1016/j.colsurfb.2020.111404.
- Chen, K.; Chang, C.; Liu, Z.; Zhou, Y.; Xu, Q.; Li, C.; Huang, Z.; Xu, H.; Xu, P.; Lu, B. Hyaluronic acid targeted and pH-responsive nanocarriers based on hollow mesoporous silica nanoparticles for chemo-photodynamic combination ther-apy. Colloids Surf. B. Biointerfaces 2020, 194, 111166, doi:10.1016/j.colsurfb.2020.111166.
- Lu, J.; Luo, B.; Chen, Z.; Yuan, Y.; Kuang, Y.; Wan, L.; Yao, L.; Chen, X.; Jiang, B.; Liu, J.; et al. Host-guest fabrication of du-al-responsive hyaluronic acid/mesoporous silica nanoparticle based drug delivery system for targeted cancer therapy. Int. J. Biol. Macromol. 2020, 146, 363–373, doi:10.1016/j.ijbiomac.2019.12.265.
- Shi, X.-L.; Li, Y.; Zhao, L.-M.; Su, L.-W.; Ding, G. Delivery of MTH1 inhibitor (TH287) and MDR1 siRNA via hyaluronic ac-id-based mesoporous silica nanoparticles for oral cancers treatment. Colloids Surf. B. Biointerfaces 2019, 173, 599–606, doi:10.1016/j.colsurfb.2018.09.076.
- Chen, C.; Sun, W.; Wang, X.; Wang, Y.; Wang, P. pH-responsive nanoreservoirs based on hyaluronic acid end-capped mes-oporous silica nanoparticles for targeted drug delivery. Int. J. Biol. Macromol. 2018, 111, 1106–1115, doi:10.1016/j.ijbiomac.2018.01.093.
- Pallares, R.M.; Agbo, P.; Liu, X.; An, D.D.; Gauny, S.S.; Zeltmann, S.E.; Minor, A.M.; Abergel, R.J. Engineering Mesoporous Silica Nanoparticles for Targeted Alpha Therapy against Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12, 40078–40084, doi:10.1021/acsami.0c11051.
- Cai, Y.; Deng, T.; Pan, Y.; Zink, J.I. Use of Ferritin Capped Mesoporous Silica Nanoparticles for Redox and pH Triggered Drug Release In Vitro and In Vivo. Adv. Funct. Mater. 2020, 30, 2002043, doi:10.1002/adfm.202002043.
- Saini, K.; Bandyopadhyaya, R. Transferrin-Conjugated Polymer-Coated Mesoporous Silica Nanoparticles Loaded with Gemcitabine for Killing Pancreatic Cancer Cells. ACS Appl. Nano Mater. 2020, 3, 229–240, doi:10.1021/acsanm.9b01921.
- Yan, H.J.; You, Y.; Li, X.J.; Liu, L.; Guo, F.Q.; Zhang, Q.L.; Liu, D.W.; Tong, Y.; Ding, S.L.; Wang, J.Y. Preparation of RGD Pep-tide/Folate Acid Double-Targeted Mesoporous Silica Nanoparticles and Its Application in Human Breast Cancer MCF-7 Cells. Front. Pharmacol. 2020, 11, 10, doi:10.3389/fphar.2020.00898.
- Zhao, F.F.; Zhang, C.; Zhao, C.G.; Gao, W.; Fan, X.B.; Wu, G.Q. A facile strategy to fabricate a pH-responsive mesoporous silica nanoparticle end-capped with amphiphilic peptides by self-assembly. Colloid Surf. B-Biointerfaces 2019, 179, 352–362, doi:10.1016/j.colsurfb.2019.03.019.
- Lee, N.K.; Park, S.S.; Ha, C.S. pH-Sensitive Drug Delivery System Based on Mesoporous Silica Modified with Poly-L-Lysine (PLL) as a Gatekeeper. J. Nanosci. Nanotechnol. 2020, 20, 6925–6934, doi:10.1166/jnn.2020.18821.
- Qin, Y.J.; Shan, X.Q.; Han, Y.; Jin, H.; Gao, Y. Study of pH-Responsive and Polyethylene Glycol-Modified Doxorubi-cin-Loaded Mesoporous Silica Nanoparticles for Drug Delivery. J. Nanosci. Nanotechnol. 2020, 20, 5997–6006, doi:10.1166/jnn.2020.17885.
- Chen, M.M.; Hu, J.X.; Bian, C.C.; Zhu, C.H.; Chen, C.; Guo, Z.J.; Zhang, Z.M.; Agyekum, G.A.; Zhang, Z.Q.; Cao, X.C. pH-Responsive and Biodegradable ZnO-Capped Mesoporous Silica Composite Nanoparticles for Drug Delivery. Materials 2020, 13, 16, doi:10.3390/ma13183950.
- Zhang, H.; Xia, Q.; Zhou, D. Albumin-gated zwitterion-stabilized mesoporous silica nanorod as a pH-responsive drug de-livery system. Colloids Surf. B Biointerfaces 2020, 193, 111107, doi:10.1016/j.colsurfb.2020.111107.
- Benova, E.; Berge-Lefranc, D.; Zelenak, V.; Almasi, M.; Huntosova, V.; Hornebecq, V. Adsorption properties, the pH-sensitive release of 5-fluorouracil and cytotoxicity studies of mesoporous silica drug delivery matrix. Appl. Surf. Sci. 2020, 504, 12, doi:10.1016/j.apsusc.2019.144028.
- Chen, Y.; Lu, W.; Guo, Y.; Zhu, Y.; Song, Y. Chitosan-Gated Fluorescent Mesoporous Silica Nanocarriers for the Real-Time Monitoring of Drug Release. Langmuir 2020, 36, 6749–6756, doi:10.1021/acs.langmuir.0c00832.
- Li, L.; Lan, S.; Ma, D. Ultrastable and Versatile Layer-by-Layer Coating Based on Kinetically Trapped Host–Guest Complex-ation for Mesoporous Silica Nanoparticles. Part. Part. Syst. Charact. 2020, 37, 2000075, doi:10.1002/ppsc.202000075.
- Zhang, L.; Wei, F.; Al-Ammari, A.; Sun, D. An optimized mesoporous silica nanosphere-based carrier system with chemi-cally removable Au nanoparticle caps for redox-stimulated and targeted drug delivery. Nanot 2020, 31, 475102, doi:10.1088/1361-6528/ab9391.
- Zid, L.; Zelenak, V.; Girman, V.; Bednarcik, J.; Zelenakova, A.; Szucsova, J.; Hornebecq, V.; Hudak, A.; Sulekova, M.; Va-hovska, L. Doxorobicin as cargo in a redox-responsive drug delivery system capped with water dispersible ZnS nanoparti-cles. Rsc Adv. 2020, 10, 15825–15835, doi:10.1039/d0ra02091e.
- Liu, J.T.; Li, Y.; Zhao, M.; Lei, Z.L.; Guo, H.; Tang, Y.P.; Yan, H. Redox-responsive hollow mesoporous silica nanoparticles constructed via host-guest interactions for controllable drug release. J. Biomater. Sci. Polym. Ed. 2020, 31, 472–490, doi:10.1080/09205063.2019.1700601.
- Shen, L.Y.; Pan, S.; Niu, D.C.; He, J.P.; Jia, X.B.; Hao, J.N.; Gu, J.L.; Zhao, W.R.; Li, P.; Li, Y.S. Facile synthesis of organosili-ca-capped mesoporous silica nanocarriers with selective redox-triggered drug release properties for safe tumor chemo-therapy. Biomater. Sci. 2019, 7, 1825–1832, doi:10.1039/c8bm01669k.
- Gao, Y.; Zhong, S.; Xu, L.; He, S.; Dou, Y.; Zhao, S.; Chen, P.; Cui, X. Mesoporous silica nanoparticles capped with graphene quantum dots as multifunctional drug carriers for photo-thermal and redox-responsive release. Microporous Mesoporous Ma-ter. 2019, 278, 130–137, doi:10.1016/j.micromeso.2018.11.030.
- Zhang, Y.; Xing, Y.; Xian, M.; Shuang, S.M.; Dong, C. Folate-targeting and bovine serum albumin-gated mesoporous silica nanoparticles as a redox-responsive carrier for epirubicin release. New J. Chem. 2019, 43, 2694–2701, doi:10.1039/c8nj05476b.
- Cai, D.F.; Hang, C.Y.; Liu, C.; Mae, X.X.; Qian, J.Y.; Zhou, J.W.; Li, Y.; Sun, Y.M.; Zhang, C.T.; Zhu, W.Q. Chitosan-capped enzyme-responsive hollow mesoporous silica nanoplatforms for colon-specific drug delivery. Nanoscale Res. Lett. 2020, 15, 13, doi:10.1186/s11671-020-03351-8.
- Paredes, K.O.; Diaz-Garcia, D.; Garcia-Almodovar, V.; Chamizo, L.L.; Marciello, M.; Diaz-Sanchez, M.; Prashar, S.; Gomez-Ruiz, S.; Filice, M. Multifunctional Silica-Based Nanoparticles with Controlled Release of Organotin Metallodrug for Targeted Theranosis of Breast Cancer. Cancers 2020, 12, 23, doi:10.3390/cancers12010187.
- Kumar, B.; Kulanthaivel, S.; Mondal, A.; Mishra, S.; Banerjee, B.; Bhaumik, A.; Banerjee, I.; Giri, S. Mesoporous silica nano-particle based enzyme responsive system for colon specific drug delivery through guar gum capping. Colloids Surf. B. Bioin-terfaces 2017, 150, 352–361, doi:10.1016/j.colsurfb.2016.10.049.
- Jiang, H.; Shi, X.; Yu, X.; He, X.; An, Y.; Lu, H. Hyaluronidase Enzyme-responsive Targeted Nanoparticles for Effective De-livery of 5-Fluorouracil in Colon Cancer. Pharm. Res. 2018, 35, 73, doi:10.1007/s11095-017-2302-4.
- Naz, S.; Wang, M.Y.; Han, Y.N.; Hu, B.; Teng, L.P.; Zhou, J.; Zhang, H.J.; Chen, J.H. Enzyme-responsive mesoporous silica nanoparticles for tumor cells and mitochondria multistage-targeted drug delivery. Int. J. Nanomed. 2019, 14, 2533–2542, doi:10.2147/ijn.S202210.
- Zhou, J.; Wang, M.; Ying, H.; Su, D.; Zhang, H.; Lu, G.; Chen, J. Extracellular Matrix Component Shelled Nanoparticles as Dual Enzyme-Responsive Drug Delivery Vehicles for Cancer Therapy. ACS Biomater. Sci. Eng. 2018, 4, 2404–2411, doi:10.1021/acsbiomaterials.8b00327.
- Qiao, H.; Jia, J.; Shen, H.; Zhao, S.; Chen, E.; Chen, W.; Di, B.; Hu, C. Capping Silica Nanoparticles with Trypto-phan-Mediated Cucurbit[8]uril Complex for Targeted Intracellular Drug Delivery Triggered by Tumor-Overexpressed IDO1 Enzyme. Adv. Healthc. Mater. 2019, 8, 1900174, doi:10.1002/adhm.201900174.
- Eskandari, P.; Bigdeli, B.; Daryasari, M.P.; Baharifar, H.; Bazri, B.; Shourian, M.; Amani, A.; Sadighi, A.; Goliaei, B.; Khoobi, M.; et al. Gold-capped mesoporous silica nanoparticles as an excellent enzyme-responsive nanocarrier for controlled doxo-rubicin delivery. J. Drug Target. 2019, 27, 1084–1093, doi:10.1080/1061186x.2019.1599379.
- Salinas, Y.; Bruggemann, O.; Monkowius, U.; Teasdale, I. Visible Light Photocleavable Ruthenium-Based Molecular Gates to Reversibly Control Release from Mesoporous Silica Nanoparticles. Nanomaterials 2020, 10, 15, doi:10.3390/nano10061030.
- Wang, M.; Wang, T.; Wang, D.; Jiang, W.; Fu, J. Acid and light stimuli-responsive mesoporous silica nanoparticles for con-trolled release. J. Mater. Sci. 2019, 54, 6199–6211, doi:10.1007/s10853-019-03325-x.
- Hernández Montoto, A.; Llopis-Lorente, A.; Gorbe, M.; M. Terrés, J.; Cao-Milán, R.; Díaz de Greñu, B.; Alfonso, M.; Ibañez, J.; Marcos, M.D.; Orzáez, M.; et al. Janus Gold Nanostars–Mesoporous Silica Nanoparticles for NIR-Light-Triggered Drug De-livery. Chem. A Eur. J. 2019, 25, 8471–8478, doi:10.1002/chem.201900750.
- Liu, Z.; Shi, J.; Wang, Y.; Gan, Y.; Wan, P. Facile preparation of pyrenemethyl ester-based nanovalve on mesoporous silica coated upconversion nanoparticle for NIR light-triggered drug release with potential monitoring capability. Colloids Surf. Physicochem. Eng. Asp. 2019, 568, 436–444, doi:10.1016/j.colsurfa.2019.02.027.
- Han, R.-L.; Shi, J.-H.; Liu, Z.-J.; Hou, Y.-F.; Wang, Y. Near-Infrared Light-Triggered Hydrophobic-to-Hydrophilic Switch Nanovalve for On-Demand Cancer Therapy. ACS Biomater. Sci. Eng. 2018, 4, 3478–3486, doi:10.1021/acsbiomaterials.8b00437.
- Li, B.; Harlepp, S.; Gensbittel, V.; Wells, C.J.R.; Bringel, O.; Goetz, J.G.; Begin-Colin, S.; Tasso, M.; Begin, D.; Mertz, D. Near infra-red light responsive carbon nanotubes@mesoporous silica for photothermia and drug delivery to cancer cells. Mater. Today Chem. 2020, 17, 100308, doi:10.1016/j.mtchem.2020.100308.
- Zhang, Q.; Chen, X.; Shi, H.; Dong, G.; Zhou, M.; Wang, T.; Xin, H. Thermo-responsive mesoporous silica/lipid bilayer hy-brid nanoparticles for doxorubicin on-demand delivery and reduced premature release. Colloids Surf. B. Biointerfaces 2017, 160, 527–534, doi:10.1016/j.colsurfb.2017.10.005.
- Tian, Z.; Yu, X.; Ruan, Z.; Zhu, M.; Zhu, Y.; Hanagata, N. Magnetic mesoporous silica nanoparticles coated with ther-mo-responsive copolymer for potential chemo- and magnetic hyperthermia therapy. Microporous Mesoporous Mater. 2018, 256, 1–9, doi:10.1016/j.micromeso.2017.07.053.
- Eltohamy, M.; Seo, J.-W.; Hwang, J.-Y.; Jang, W.-C.; Kim, H.-W.; Shin, U.S. Ionic and thermo-switchable polymer-masked mesoporous silica drug-nanocarrier: High drug loading capacity at 10°C and fast drug release completion at 40 °C. Colloids Surf. B. Biointerfaces 2016, 144, 229–237, doi:10.1016/j.colsurfb.2016.04.023.
- Kamachi, Y.; Bastakoti, B.P.; Alshehri, S.M.; Miyamoto, N.; Nakato, T.; Yamauchi, Y. Thermo-responsive hydrogels contain-ing mesoporous silica toward controlled and sustainable releases. MatL 2016, 168, 176–179, doi:10.1016/j.matlet.2015.12.132.
- Iturrioz-Rodriguez, N.; Correa-Duarte, M.A.; Fanarraga, M.L. Controlled drug delivery systems for cancer based on meso-porous silica nanoparticles. Int. J. Nanomed. 2019, 14, 3389–3401, doi:10.2147/IJN.S198848.
- Chen, C.; Ma, T.; Tang, W.; Wang, X.; Wang, Y.; Zhuang, J.; Zhu, Y.; Wang, P. Reversibly-regulated drug release using poly(tannic acid) fabricated nanocarriers for reduced secondary side effects in tumor therapy. Nanoscale Horiz 2020, 5, 986–998, doi:10.1039/D0NH00032A.
- Li, R.; Peng, F.; Cai, J.; Yang, D.; Zhang, P. Redox dual-stimuli responsive drug delivery systems for improving tumor-targeting ability and reducing adverse side effects. Asian J. Pharm. Sci. 2020, 15, 311–325, doi:10.1016/j.ajps.2019.06.003.
- Zhang, L.; Wei, F.; Al-Ammari, A.; Sun, D. An optimized mesoporous silica nanosphere-based carrier system with chemically removable Au nanoparticle caps for redox-stimulated and targeted drug delivery. Nanot 2020, 31, 475102, doi:10.1088/1361-6528/ab9391.
- Li, L.; Lan, S.; Ma, D. Ultrastable and Versatile Layer-by-Layer Coating Based on Kinetically Trapped Host–Guest Complexation for Mesoporous Silica Nanoparticles. Part. Part. Syst. Charact. 2020, 37, 2000075, doi:10.1002/ppsc.202000075.
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. “Smart” materials-based near-infrared light-responsive drug deliv-ery systems for cancer treatment: A review. J. Mater. Res. Technol. 2019, 8, 1497–1509, doi:10.1016/j.jmrt.2018.03.007.
- Ghosh Dastidar, D.; Chakrabarti, G. Chapter 6-Thermoresponsive Drug Delivery Systems, Characterization and Applica-tion. In Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 133–155. doi:10.1016/B978-0-12-814029-1.00006-5.
- Xu, Y.Q.; Xiao, L.Y.; Chang, Y.T.; Cao, Y.A.; Chen, C.G.; Wang, D. pH and Redox Dual-Responsive MSN-S-S-CS as a Drug Delivery System in Cancer Therapy. Materials 2020, 13, 12, doi:10.3390/ma13061279.
- Chen, C.; Yao, W.; Sun, W.; Guo, T.; Lv, H.; Wang, X.; Ying, H.; Wang, Y.; Wang, P. A self-targeting and controllable drug delivery system constituting mesoporous silica nanoparticles fabricated with a multi-stimuli responsive chitosan-based thin film layer. Int. J. Biol. Macromol. 2019, 122, 1090–1099, doi:10.1016/j.ijbiomac.2018.09.058.
- Zhang, K.; Ding, C.; Liu, X.; Gao, J.; Wu, D.; Qin, Y.; Kong, Y. A redox and pH dual-triggered drug delivery platform based on chitosan grafted tubular mesoporous silica. Ceram. Int. 2019, 45, 22603–22609, doi:10.1016/j.ceramint.2019.07.292.
- Song, Y.Y.; Cai, L.; Tian, Z.C.; Wu, Y.; Chen, J. Phytochemical Curcumin-Coformulated, Silver-Decorated Melanin-like Pol-ydopamine/Mesoporous Silica Composites with Improved Antibacterial and Chemotherapeutic Effects against Drug-Resistant Cancer Cells. ACS Omega 2020, 5, 15083–15094, doi:10.1021/acsomega.0c00912.
- Li, X.; Hu, S.; Lin, Z.; Yi, J.; Liu, X.; Tang, X.; Wu, Q.; Zhang, G. Dual-responsive mesoporous silica nanoparticles coated with carbon dots and polymers for drug encapsulation and delivery. Nanomedicine 2020, 15, 2447–2458, doi:10.2217/nnm-2019-0440.
- Chen, Q.; Chen, Y.; Zhang, W.; Huang, Q.; Hu, M.; Peng, D.; Peng, C.; Wang, L.; Chen, W. Acidity and Glutathione Du-al-Responsive Polydopamine-Coated Organic-Inorganic Hybrid Hollow Mesoporous Silica Nanoparticles for Controlled Drug Delivery. ChemMedChem 2020, 15, 1940–1946, doi:10.1002/cmdc.202000263.
- Bhavsar, D.B.; Patel, V.; Sawant, K.K. Design and characterization of dual responsive mesoporous silica nanoparticles for breast cancer targeted therapy. Eur. J. Pharm. Sci. 2020, 152, 105428, doi:10.1016/j.ejps.2020.105428.
- Wan, L.H.; Chen, Z.Y.; Deng, Y.; Liao, T.; Kuang, Y.; Liu, J.; Duan, J.L.; Xu, Z.Q.; Jiang, B.B.; Li, C. A novel intratumoral pH/redox-dual-responsive nanoplatform for cancer MR imaging and therapy. J. Colloid Interface Sci. 2020, 573, 263–277, doi:10.1016/j.jcis.2020.04.026.
- Wang, W.X.; Wang, Y.Y.; Wang, Y.; Gong, H.M.; Zhu, H.D.; Liu, M.X. Redox/pH dual stimuli-responsive ZnO QDs-gated mesoporous silica nanoparticles as carriers in cancer therapy. IET Nanobiotechnol. 2019, 13, 640–649, doi:10.1049/iet-nbt.2019.0031.
- Lu, J.; Chen, Q.; Ding, X.; Wen, J.; Zhang, Y.; Li, H.; Xu, Y.; Liu, F.; Chen, S.-S.; Sun, S. BSA modified, disulfide-bridged mes-oporous silica with low biotoxicity for dual-responsive drug delivery. Microporous Mesoporous Mater. 2019, 278, 257–266, doi:10.1016/j.micromeso.2018.12.001.
- Yuan, N.-n.; Li, S.-j.; Li, G.-q. Sodium alginate coated mesoporous silica for dual bio-responsive controlled drug delivery. J. Drug Deliv. Sci. Technol. 2018, 46, 348–353, doi:10.1016/j.jddst.2018.05.026.
- Ding, X.; Yu, W.; Wan, Y.; Yang, M.; Hua, C.; Peng, N.; Liu, Y. A pH/ROS-responsive, tumor-targeted drug delivery system based on carboxymethyl chitin gated hollow mesoporous silica nanoparticles for anti-tumor chemotherapy. Carbohydr. Polym. 2020, 245, 116493, doi:10.1016/j.carbpol.2020.116493.
- Salinas, Y.; Kneidinger, M.; Fornaguera, C.; Borros, S.; Bruggemann, O.; Teasdale, I. Dual stimuli-responsive polyphos-phazene-based molecular gates for controlled drug delivery in lung cancer cells. Rsc Adv. 2020, 10, 27305–27314, doi:10.1039/d0ra03210g.
- Wang, J.; Huang, N.; Peng, Q.; Cheng, X.; Li, W. Temperature/pH dual-responsive and luminescent drug carrier based on PNIPAM-MAA/lanthanide-polyoxometalates for controlled drug delivery and imaging in HeLa cells. MCP 2020, 239, 121994, doi:10.1016/j.matchemphys.2019.121994.
- Song, Z.; Liu, Y.; Shi, J.; Ma, T.; Zhang, Z.; Ma, H.; Cao, S. Hydroxyapatite/mesoporous silica coated gold nanorods with im-proved degradability as a multi-responsive drug delivery platform. Mater. Sci. Eng. C 2018, 83, 90–98, doi:10.1016/j.msec.2017.11.012.
- Abedi, M.; Abolmaali, S.S.; Abedanzadeh, M.; Borandeh, S.; Samani, S.M.; Tamaddon, A.M. Citric acid functionalized silane coupling versus post-grafting strategy for dual pH and saline responsive delivery of cisplatin by Fe3O4/carboxyl function-alized mesoporous SiO2 hybrid nanoparticles: A-synthesis, physicochemical and biological characterization. Mater. Sci. Eng. C 2019, 104, 109922, doi:10.1016/j.msec.2019.109922.
- Zhu, D.D.; Hu, C.L.; Liu, Y.; Chen, F.; Zheng, Z.; Wang, X.L. Enzyme-/Redox-Responsive Mesoporous Silica Nanoparticles Based on Functionalized Dopamine as Nanocarriers for Cancer Therapy. ACS Omega 2019, 4, 6097–6105, doi:10.1021/acsomega.8b02537.
- Bahrami, F.; Abdekhodaie, M.J.; Behroozi, F.; Mehrvar, M. Nano mesoporous silica for cancer treatment: ROS-responsive and redox-responsive carriers. J. Drug Deliv. Sci. Technol. 2020, 57, 101510, doi:10.1016/j.jddst.2020.101510.
- Cui, Y.; Deng, R.; Li, X.; Wang, X.; Jia, Q.; Bertrand, E.; Meguellati, K.; Yang, Y.-W. Temperature-sensitive polypeptide brushes-coated mesoporous silica nanoparticles for dual-responsive drug release. Chin. Chem. Lett. 2019, 30, 2291–2294, doi:10.1016/j.cclet.2019.08.017.
- Wu, Y.; Xu, Z.; Sun, W.; Yang, Y.; Jin, H.; Qiu, L.; Chen, J.; Chen, J. Co-responsive smart cyclodextrin-gated mesoporous sili-ca nanoparticles with ligand-receptor engagement for anti-cancer treatment. Mater. Sci. Eng. C 2019, 103, 109831, doi:10.1016/j.msec.2019.109831.
- Guo, F.; Li, G.Y.; Zhou, H.Q.; Ma, S.M.; Guo, L.; Liu, X.Y. Temperature and H2O2-operated nano-valves on mesoporous silica nanoparticles for controlled drug release and kinetics. Colloid Surf. B-Biointerfaces 2020, 187, 8, doi:10.1016/j.colsurfb.2019.110643.
- Chen, Y.; Zhao, Y.; Sun, L.; Zou, X. Tri-responsive porous silica carrier with gold nanoparticles for chemophotothermal combination therapy. JSGST 2020, 93, 332–340, doi:10.1007/s10971-019-05183-0.
- Zhang, R.-Q.; Liu, Z.-Q.; Luo, Y.-L.; Xu, F.; Chen, Y.-S. Tri-stimuli responsive carbon nanotubes covered by mesoporous silica graft copolymer multifunctional materials for intracellular drug delivery. J. Ind. Eng. Chem. 2019, 80, 431–443, doi:10.1016/j.jiec.2019.08.023.
- Lei, W.; Sun, C.; Jiang, T.; Gao, Y.; Yang, Y.; Zhao, Q.; Wang, S. Polydopamine-coated mesoporous silica nanoparticles for multi-responsive drug delivery and combined chemo-photothermal therapy. Mater. Sci. Eng. C 2019, 105, 110103, doi:10.1016/j.msec.2019.110103.
- Lu, H.; Zhao, Q.; Wang, X.; Mao, Y.; Chen, C.; Gao, Y.; Sun, C.; Wang, S. Multi-stimuli responsive mesoporous silica-coated carbon nanoparticles for chemo-photothermal therapy of tumor. Colloids Surf. B. Biointerfaces 2020, 190, 110941, doi:10.1016/j.colsurfb.2020.110941.
- Zhang, R.-Q.; Liu, Z.-Q.; Luo, Y.-L.; Xu, F.; Chen, Y.-S. Tri-stimuli responsive carbon nanotubes covered by mesoporous sili-ca graft copolymer multifunctional materials for intracellular drug delivery. J. Ind. Eng. Chem. 2019, 80, 431–443, doi:10.1016/j.jiec.2019.08.023.
- Meng, Q.Y.; Cong, H.L.; Hu, H.; Xu, F.J. Rational design and latest advances of codelivery systems for cancer therapy. Mater. Today Bio 2020, 7, 100056, doi:10.1016/j.mtbio.2020.100056.
- Zhang, J.; Weng, L.; Su, X.; Lu, G.; Liu, W.; Tang, Y.; Zhang, Y.; Wen, J.; Teng, Z.; Wang, L. Cisplatin and doxorubicin high-loaded nanodrug based on biocompatible thioether- and ethane-bridged hollow mesoporous organosilica nanoparti-cles. J. Colloid Interface Sci. 2018, 513, 214–221, doi:10.1016/j.jcis.2017.10.116.
- Pan, Y.; Zhou, S.; Li, Y.; Parshad, B.; Li, W.; Haag, R. Novel dendritic polyglycerol-conjugated, mesoporous silica-based tar-geting nanocarriers for co-delivery of doxorubicin and tariquidar to overcome multidrug resistance in breast cancer stem cells. J. Control. Release 2020, 10.1016/j.jconrel.2020.11.015, doi:10.1016/j.jconrel.2020.11.015.
- Chen, X.; Song, L.; Li, X.; Zhang, L.; Li, L.; Zhang, X.; Wang, C. Co-delivery of hydrophilic/hydrophobic drugs by multifunc-tional yolk-shell nanoparticles for hepatocellular carcinoma theranostics. Chem. Eng. J. 2020, 389, 124416, doi:10.1016/j.cej.2020.124416.
- Ali, O.M.; Bekhit, A.A.; Khattab, S.N.; Helmy, M.W.; Abdel-Ghany, Y.S.; Teleb, M.; Elzoghby, A.O. Synthesis of lactoferrin mesoporous silica nanoparticles for pemetrexed/ellagic acid synergistic breast cancer therapy. Colloids Surf. B. Biointerfaces 2020, 188, 110824, doi:10.1016/j.colsurfb.2020.110824.
- Xing, Y.; Zhou, Y.; Zhang, Y.; Zhang, C.; Deng, X.; Dong, C.; Shuang, S. Facile Fabrication Route of Janus Gold-Mesoporous Silica Nanocarriers with Dual-Drug Delivery for Tumor Therapy. ACS Biomater. Sci. Eng. 2020, 6, 1573–1581, doi:10.1021/acsbiomaterials.0c00042.
- Salinas, Y.; Kneidinger, M.; Fornaguera, C.; Borros, S.; Bruggemann, O.; Teasdale, I. Dual stimuli-responsive polyphos-phazene-based molecular gates for controlled drug delivery in lung cancer cells. Rsc Adv. 2020, 10, 27305–27314, doi:10.1039/d0ra03210g.
- Zhang, R.-Q.; Liu, Z.-Q.; Luo, Y.-L.; Xu, F.; Chen, Y.-S. Tri-stimuli responsive carbon nanotubes covered by mesoporous silica graft copolymer multifunctional materials for intracellular drug delivery. J. Ind. Eng. Chem. 2019, 80, 431–443, doi:10.1016/j.jiec.2019.08.023.
