Docosahexaenoic acid (DHA) supplementation during pregnancy has been recommended by several health organizations due to its role in neural, visual, and cognitive development. There are several fat sources available on the market for the manufacture of these dietary supplements with DHA. These fat sources differ in the lipid structure in which DHA is esterified, mainly phospholipids (PL) and triglycerides (TG) molecules. The supplementation of DHA in the form of PL or TG during pregnancy can lead to controversial results depending on the animal model, physiological status and the fat sources utilized. The intestinal digestion, placental uptake, and fetal accretion of DHA may vary depending on the lipid source of DHA ingested by the mother. The form of DHA used in maternal supplementation that would provide an optimal DHA accretion for fetal brain development, based on the available data obtained most of them from different animal models, indicates no consistent differences in fetal accretion when DHA is provided as TG or PL. Other related lipid species are under evaluation, e.g., lyso-phospholipids, with promising results to improve DHA bioavailability although more studies are needed. In this review, the evidence on DHA bioavailability and accumulation in both maternal and fetal tissues after the administration of DHA supplementation during pregnancy in the form of PL or TG in different models is summarized.
- DHA
- Pregnancy
- supplementation
- phospholipids
- triglycerides
1. Introduction
There is a growing interest in the effects of maternal diet consumed during pregnancy on both development and fetal programming of many physiological functions. During pregnancy and lactation there is an elevated docosahexaenoic acid (22:6 omega-3, DHA) requirement in the fetus and neonate as it is a critical building block of brain and retina [1,2,3]. In the last trimester of pregnancy, it is estimated a fetal accretion of 67 mg of omega-3 fatty acids (FA) per day, mainly DHA, and around 5% is delivered to the brain (3.1 mg/d) [4,5]. DHA conversion efficiency from α-linolenic acid (18:3 omega-3), its essential FA precursor, is very low (<1%) in fetus, placenta and newborns [6,7,8,9], being therefore insufficient to satisfy the high supply of DHA needed by the growing fetus [10,11]. Moreover, several studies have shown that supplementation with α-linolenic acid in human adults is not a good strategy to increase DHA levels, being necessary the direct supplementation with the preformed DHA molecule to observe: enhanced DHA status in blood and tissues [12], higher transfer of DHA to the fetus [13] or even to increase DHA secretion in human milk [14].
2. DHA Recommendations and Health Outcomes
2.1. DHA Intake during the Perinatal Period
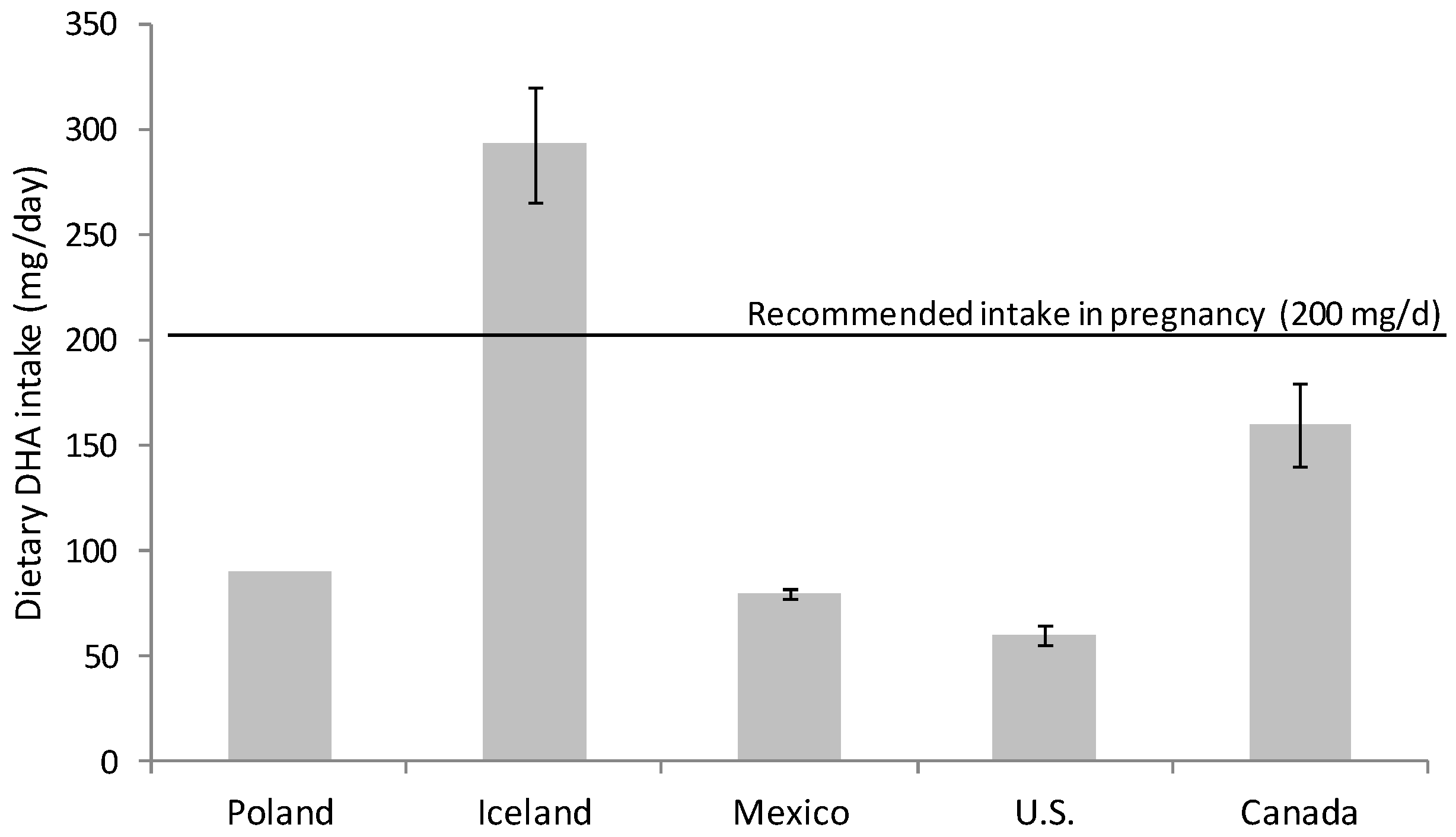
Omega-3 FA intake had fallen during the 20th century; the development of the modern vegetable oil industry, the use of cereal grains and the change in eating habits have produced a remarkable disparity in the ratio of consumption of omega-6 and omega-3 FA [15,16]. Omega-6 FA consumption, mainly in the form of linoleic acid (18:2 n-6), has increased at the expense of omega-3 FA (DHA and eicosapentaenoic acid (EPA, 20:5 n-3)) in the general population [15,16,17]. Nowadays, the intake of DHA in developed countries with free access to food of animal origin rich in micronutrients and omega-3 FA is highly variable. There are several studies evaluating DHA consumption that warn about inadequate dietary DHA intake for many women during pregnancy [18,19,20] (Figure 1). It is especially alarming the situation in western countries, for example in Canada and U.S. where DHA intakes are especially low, revealing that a majority of childbearing-age and pregnant women consume less than the recommended DHA dose [21,22] (Figure 1).
The preferential placental uptake and transfer of DHA to the fetus in relation to other FA (palmitic, oleic and linoleic acid) has been demonstrated by the administration of stable isotope-labelled FAs to pregnant women [25,26]. Moreover, the percentage of DHA and arachidonic acid (AA; 20:4 omega-6,) both in plasma and adipose tissue is higher in the neonate than in the mother, which reveals the important role of the placenta in the concentration of these FA in the fetal compartment [27,28]. This process is known as “biomagnification” and is defined as selective enrichment of these FA in fetal, with respect to maternal plasma [29]. AA and DHA concentration in non-esterified FA (NEFA) of the intervillous space of the placenta is 3–4 times higher than in maternal blood outside the placenta [30]. This fact implies that there is certain selectivity of placental tissue for the release of these long-chain polyunsaturated FA (LC-PUFA) from the circulating lipoproteins.
It is well known that the maternal DHA intake, and hence maternal DHA levels, during pregnancy determines the DHA status of the newborn at birth and for several weeks following delivery [31,32,33,34]. Large observational studies have shown that women with low seafood intakes during pregnancy are prone to an increased risk of poor infant cognition and behavioral outcome [35,36]. Low levels of DHA and AA in maternal plasma and cord blood has been related to lower head circumference, lower birthweight, lower placental weight [32], and less cognitive and visual maturation during childhood [37,38]. Other studies found associations between omega-3 FA intake during pregnancy and lower risks of intrauterine growth restriction, preterm birth, allergies, and asthma in children [19,39,40]. However, some randomized controlled trials and meta-analysis reported inconsistent evidences and very few differences between child born from omega-3 supplemented vs. placebo mothers on long-term vision, growth and neurodevelopment outcomes [41,42,43,44,45]. Further follow-up studies are needed to assess the longer-term consequences and health outcomes for both mother and child of maternal omega-3 supplementation.
2.2. Dietary Recommendation during Pregnancy and Lactation
DHA dietary supplementation has been recommended by several health organizations [46,47]. European and global guidelines recommends the intake of at least 200 mg/d DHA during these periods, which can be met with two servings of fish per week [48,49,50]. The highest concentration of DHA is found in seafood, especially in oily fish (tuna, salmon, herring, mackerel, etc.) [51]. Smaller fishes are highly recommendable since they contain lower levels of methyl mercury and other contaminants than large-size predators [49]. Probably, the dose should be higher to detect significant effects on some outcomes but, due to the high variability in DHA intake from other sources, these recommendations are highly conservative.
DHA supplementation should be considered only if dietary consumption (natural sources) is not sufficient to meet the recommendations or when it is problematic due to food availability, socio-cultural dietary preferences, fish aversion, ethics issues (e.g., vegans), or other factors [52].
3. Conclusions
The animal model used to evaluate the materno-fetal transfer of DHA as PL or TG is a key issue since placental structure differs among the species used for such studies. Administration of DHA-rich PL produces a modest enrichment of DHA in PL plasma lipid fraction in piglets and pregnant sows compared to DHA-TG administration while similar or opposite results have been observed in other species. Intestinal digestion, re-esterification in both gut enterocytes and liver, as well as placental transfer processes reduce the impact of the dietary intervention with different lipid sources on fetal DHA levels. Dietary lipid utilization and bioavailability comprises several metabolic processes that are not completely understood and further research is needed. There are a limited number of studies evaluating placental and fetal accretion of DHA after the administration of different fat sources to pregnant animals (PL and TG). Despite some differences observed in placental DHA content between animal species, fetal DHA accretion and, especially, fetal brain DHA accumulation after PL or TG administration was similar. However, it is important to note that the use of animal models (rodents and pigs) in most studies might have some limitations in extrapolating results to humans. Lyso-PL have been proposed as a preferred physiological carrier of DHA to the brain, the available data on DHA Lyso-PL bioavailability with respect to other sources are promising and seem to indicate an increased DHA incorporation in some tissues but more studies are needed to evaluate their effects during pregnancy, fetal bioavailability, and long-term effects on neurodevelopment. In summary, although most of the results available were obtained in animal models, both PL and TG sources can be used for the manufacture of DHA supplements during pregnancy since they show a comparable bioavailability and promote similar DHA accretion in the fetus. The dose of DHA administered is perhaps more decisive than the fat source to increase fetal DHA status.
This entry is adapted from the peer-reviewed paper 10.3390/nu13020511
