Rice grain yield is a complex trait determined by three components: panicle number, grain number per panicle (GNPP) and grain weight. GNPP is the major contributor to grain yield and is crucial for its improvement. GNPP is determined by a series of physiological and biochemical steps, including inflorescence development, formation of rachis branches such as primary rachis branches and secondary rachis branches, and spikelet specialisation (lateral and terminal spikelets).
- GNPP
- grain yield
- phase transition
- rachis branch
- rice panicle
- spikelet specialisation
1. Introduction
Rice grain yield is primarily determined by three traits—grain number per panicle (GNPP), grain weight and number of panicles [1]. Because the rice grain yield per unit area is high, increasing the GNPP could further improve the grain yield [1–3].
2. Panicle Development and GNPP Determination in Rice
2.1. Panicle Development
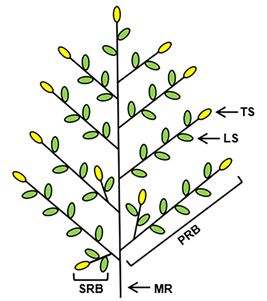
The rice panicle is composed of the main rachis, rachis branches (primary rachis branches (PRBs) and secondary rachis branches (SRBs) and spikelets (lateral and terminal spikelets) (Figure 1) [4,5]. In a few rice varieties, there are tertiary rachis branches in the panicle. GNPP determination involves the development of the inflorescence, formation of rachis branches and spikelet specialisation. During rice panicle development, the inflorescence meristem (IM) is an important regulator of GNPP formation [6]. In rice, transition to the reproductive phase involves the transformation of the shoot apical meristem (SAM) into the IM, initiating the growth of several lateral meristems as PRBs. Next, the IM loses its activity, leaving a vestige at the base of the uppermost PRB. The PRB meristem produces next-order branches as lateral meristems. The few initially formed lateral meristems grow as SRBs and later meristems directly from spikelet meristems. Lateral spikelets differentiate directly from newly formed lateral meristems, and terminal spikelets are converted from rachis branch meristems. Therefore, three factors—rachis branch formation, the transition from rachis branch meristem to spikelet meristem and spikelet specialisation—determine the overall architecture of the panicle and the GNPP in rice [7].
Figure 1. Panicle architecture of rice. The green ellipses show the lateral spikelets and the yellow ellipses show the terminal spikelets. LS, lateral spikelet; MR, main rachis; PRB, primary rachis branch; SRB, secondary rachis branch; TS, terminal spikelet.
2.2. GNPP Determination in Rice
Plant hormones, such as auxin, gibberellin (GA), cytokinin (CK), abscisic acid (ABA) and ethylene, are involved in regulating panicle development and GNPP in rice [8,9]. Auxin has a pivotal role in panicle development, as it is required for the initiation and maintenance of axillary meristems. Auxin is produced mainly in growing shoot apices and is transported basipetally down the site along specific transport routes through polar transport machinery. Consequently, disruption in auxin synthesis or auxin transport results in fewer rachis branches and reduced GNPP in rice [10–12]. GA can affect panicle-associated traits including panicle length, rachis branch number and GNPP in rice [13]. A previous study demonstrated that OsCYP71D8L controls panicle-related traits by regulating GA homeostasis. Gain-of-function of OsCYP71D8L leads to shorter panicles, fewer rachis branches, and reduced GNPP in rice [14]. It has been reported that the fine-tuning of bioactive CK level in the IM is a critical trait for controlling the number of rachis branches and GNPP in rice. The decreased level of bioactive CK in rice IM is usually accompanied by fewer rachis branches and reduced GNPP [15,16], and the weakened CK signalling in rice IM also results in fewer rachis branches and reduced GNPP [17,18]. This evidence suggests that CK positively regulates GNPP in rice. On the other hand, the stress hormones such as ABA and ethylene negatively regulate the GNPP in rice [9,19]. In addition, signalling cascades and responses of several plant hormones overlap, and the molecular components are often shared among them. A complex network of effectors of multiple hormonal pathways collide and communicate to regulate critical agronomic traits including GNPP in rice [8].
Increasing evidence indicates that plant hormones mediate GNPP determination mainly through the transcriptional or post-transcriptional regulation of GNPP-related genes in rice [8,20,21]. Additionally, GNPP-related genes control panicle development mainly by regulating three factors, including rachis branch formation, the transition from rachis branch meristem to spikelet meristem and spikelet specialisation [7,15,16].
Reference
1. Zhou, Y.; Tao, Y.; Yuan, Y.; Zhang, Y.; Miao, J.; Zhang, R.; Yi, C.; Gong, Z.; Yang, Z.; Liang, G. Characterisation of a novel quantitative trait locus, GN4-1, for grain number and yield in rice (Oryza sativa L.). Theor. Appl. Genet. 2018, 131, 637–648.
2. Chen, H.; Tang, Y.; Liu, J.; Tan, L.; Jiang, J.; Wang, M.; Zhu, Z.; Sun, X.; Sun, C. Emergence of a novel chimeric gene underlying grain number in rice. Genetics 2017, 205, 993–1002.
3. Chen, L.; Bian, J.; Shi, S.; Yu, J.; Khanzada, H.; Wassan, G.M.; Zhu, C.; Luo, X.; Tong, S.; Yang, X.; et al. Genetic analysis for the grain number heterosis of a super-hybrid rice WFYT025 combination using RNA-Seq. Rice 2018, 11, 37.
4. Lu, H.; Shi, Z. Molecular research progress of rice panicle development. Plant Physiol. J. 2013, 49, 111–121.
5. Zhu, K.; Tao, H.; Min, C.; Yang, Y. Research progress on rice panicle development. Mol. Plant Breed. 2015, 13, 2109–2117.
6. Li, S.; Zhao, B.; Yuan, D.; Duan, M.; Qian, Q.; Tang, L.; Wang, B.; Liu, X.; Zhang, J.; Wang, J.; et al. Rice zinc finger protein DST enhances grain production through controlling Gn1a/OsCKX2 expression. Proc. Natl. Acad. Sci. USA 2013, 110, 3167–3172.
7. Ikeda-Kawakatsu, K.; Yasuno, N.; Oikawa, T.; Iida, S.; Nagato, Y.; Maekawa, M.; Kyozuka, J. Expression level of ABERRANT PANICLE ORGANIZATION1 determines rice inflorescence form through control of cell proliferation in the meristem. Plant Physiol. 2009, 150, 736–747.
8. Deveshwar, P.; Prusty, A.; Sharma, S.; Tyagi, A.K. Phytohormone-Mediated molecular mechanisms involving multiple genes and QTL govern grain number in rice. Front. Genet. 2020, 11, 586462.
9. Miao, C.; Xiao, L.; Hua, K.; Zou, C.; Zhao, Y.; Bressan, R.A.; Zhu, J.-K. Mutations in a subfamily of abscisic acid receptor genes promote rice growth and productivity. Proc. Natl. Acad. Sci. USA 2018, 115, 6058–6063.
10. Yoshida, A.; Ohmori, Y.; Kitano, H.; Taguchi-Shiobara, F.; Hirano, H.Y. Aberrant spikelet and panicle1, encoding a TOPLESS-related transcriptional co-repressor, is involved in the regulation of meristem fate in rice. Plant J. 2012, 70, 327–339.
11. Li, Y.; Zhu, J.; Wu, L.; Shao, Y.; Wu, Y.; Mao, C. Functional divergence of PIN1 paralogous genes in rice. Plant Cell Physiol. 2019, 60, 2720–2732.
12. Malik, N.; Ranjan, R.; Parida, S.K.; Agarwal, P.; Tyagi, A.K. Mediator subunit OsMED14_1 plays an important role in rice development. Plant J. 2020, 101, 1411–1429.
13. Gao, S.; Chu, C. Gibberellin metabolism and signaling: Targets for improving agronomic performance of crops. Plant Cell Physiol. 2020, 61, 1902–1911.
14. Zhou, J.; Li, Z.; Xiao, G.; Zhai, M.; Pan, X.; Huang, R.; Zhang, H. CYP71D8L is a key regulator involved in growth and stress responses by mediating gibberellin homeostasis in rice. Science 2020, 71, 1160–1170.
15. Ashikari, M.; Sakakibara, H.; Lin, S.; Yamamoto, T.; Takashi, T.; Nishimura, A.; Angeles, E.R.; Qian, Q.; Kitano, H.; Matsuoka, M. Cytokinin oxidase regulates rice grain production. Science 2005, 309, 741–745.
16. Kurakawa, T.; Ueda, N.; Maekawa, M.; Kobayashi, K.; Kojima, M.; Nagato, Y.; Sakakibara, H.; Kyozuka, J. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 2007, 445, 652–655.
17. Sun, L.; Zhang, Q.; Wu, J.; Zhang, L.; Jiao, X.; Zhang, S.; Zhang, Z.; Sun, D.; Lu, T.; Sun, Y. Two rice authentic histidine phosphotransfer proteins, OsAHP1 and OsAHP2, mediate cytokinin signaling and stress responses in rice. Plant Physiol. 2014, 165, 335–345.
18. Hirose, N.; Makita, N.; Kojima, M.; Kamada-Nobusada, T.; Sakakibara, H. Overexpression of a type-A response regulator alters rice morphology and cytokinin metabolism. Plant Cell Physiol. 2007, 48, 523–539.
19. Wuriyanghan, H.; Zhang, B.; Cao, W.-H.; Ma, B.; Lei, G.; Liu, Y.-F.; Wei, W.; Wu, H.-J.; Chen, L.-J.; Chen, H.W.; et al. The ethylene receptor ETR2 delays floral transition and affects starch accumulation in rice. Plant Cell 2009, 21, 1473–1494.
20. Sentoku, N.; Sato, Y.; Kurata, N.; Ito, Y.; Kitano, H.; Matsuoka, M. Regional expression of the Rice KN1-Type homeobox gene family during embryo, shoot, and flower development. Plant Cell 1999, 11, 1651–1664.
21. Sakamoto, T.; Sakakibara, H.; Kojima, M.; Yamamoto, Y.; Nagasaki, H.; Inukai, Y.; Sato, Y.; Matsuoka, M. Ectopic expression of KNOTTED1-like homeobox protein induces expression of cytokinin biosynthesis genes in rice. Plant Physiol. 2006, 142, 54–62.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22020728