Titanium and its compounds are broadly used in both industrial and domestic products, including jet engines, missiles, prostheses, implants, pigments, cosmetics, food, and photocatalysts for environmental purification and solar energy conversion. Although titanium/titania-containing materials are usually safe for human, animals and environment, increasing concerns on their negative impacts have been postulated. We have reported the state of knowledge about toxicity of titanium, its alloys and oxides. Due to the alarming increase in titania/titanium applications in various daily care products and medical treatment (e.g. dental implants) the possible toxicity and environmental impact should be considered. The collected data might allow to identify some harms associated with using of titania and titanium compounds.
- titania compounds
- toxicity
- titanium implants
- sea pollution
- sunscreen
1. Introduction
Titanium (Ti) is a transition metal with silver colour, high strength and low density. The most important property of titanium is its high chemical stability, i.e., titanium is even resistant to corrosion in sea water, chlorine and aqua regia. Naturally, titanium appears widely in the Earth’s crust and lithosphere, mainly in minerals, e.g., ilmenite, rutile and titanite. Metallic titanium is extracted from mineral ores mainly by Kroll and Hunter processes, i.e., by the reduction of titanium tetrachloride with magnesium and sodium, respectively. Titanium(IV) oxide (titania) is the most common titanium compound, widely used as a pigment and a photocatalyst. Other important titanium compounds are titanium chlorides, i.e., (i) titanium(IV) chloride (TiCl4), used as smoke screens and catalysts [1], and (ii) titanium(III) chloride (TiCl3), a catalyst for polypropylene synthesis [2].
Titanium added to iron, aluminium, vanadium, molybdenum, tantalum and other metals forms lightweight and strong alloys, commonly used in aerospace (jet engines, spacecraft and missiles), metallurgy processes, dental/medical applications (prostheses, orthopedic implants, dental and endodontic instruments and files, dental implants), the car industry, agriculture, the military, sporting goods, mobile phones, jewellery and other applications [3][4][5], as shown in Figure 1. For example, Ouyang et al., (2019) have shown that Ti–Mg metal–metal composites facilitate osteoconduction and osseointegration (significantly higher around Ti-Mg than that around Ti implants) for orthopedic application [6]. Similarly, increased osseointegration has been observed on Ti35Zr28Nb alloy than that on pure titanium [7]. Moreover, Maharubin et al. [8] have proven that addition of silver (0.5–2.0 wt%) to titanium might limit post-surgery infection, one of the main causes of orthopedic implant failure. Additionally, titanium has been combined with other materials/compounds, such as organic compounds and polymers. For example, tannic acid-Ti/polysulfone membranes have been recommended for water remediation, especially for textile wastewater treatment, due to high hydrophilicity, excellent antifouling ability, powerful antimicrobial capability and good long-term stability [9]. Although, titanium and titania have already been used for various applications, their toxicity has not been addressed in detail, considering the direct and indirect impacts as well as the acute and chronic toxicity.
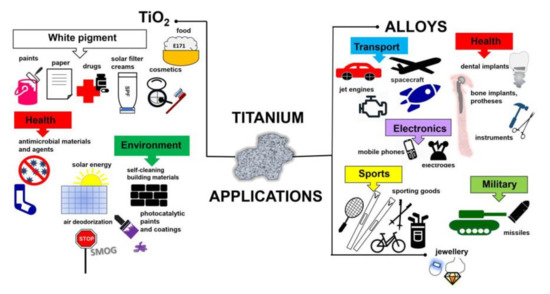
Figure 1. Schematic drawing showing miscellaneous applications of titanium-containing materials.
2. Toxicity of Titanium and Its Alloys
Titanium (Ti) has been widely used for building materials, parts of vehicles, and consumer goods (e.g., glass, camera and watches), cosmetics, drugs and dental/medical implants, due to its stability, low-density, mechanical strength, corrosion resistance and biocompatibility. Although some metals are essential biological elements, titanium has not played a biological role inside cells [10]. Moreover, it is widely known that titanium rarely causes allergic reactions in comparison to other metals. Osseointegration (binding between bone and titanium implant) without rejection was first reported by Branemark in 1983 [11]. Since this great discovery, titanium implant therapy has been developed intensively, e.g., for dental implantation, artificial joints and bones. It has been proposed that the formation of a passive film on the surface of titanium, due to the instantaneous binding of oxygen is the main reason for its lower allergic effect (lower ion release) than in the case of other metals [12][13][14].
The interest in Ti properties has been continuously growing, because of its use as an inert bio-implant material for medical and dental applications [15][16][17][18][19]. The evidence of titanium toxicity has not been reported for many years, and thus titanium has been considered as an inert material with high biocompatibility. However, in rare cases, allergic symptoms, caused by titanium (alloy) implants, have been suggested, e.g., irritation, inflammation, erythema, lichenoid reactions and so on [20][21][22][23]. Additionally, it has been reported that titanium could corrode under some conditions, e.g., low pH, in the presence of fluorine, or in the contact with other metals [14][20][23]. Interestingly, Hanawa (2004) has suggested that a release of metal ions does not necessarily damage the human body, however, their binding to biomolecules could be toxic [13]. It is known that Ti ions exhibit high activity, reacting with hydroxyl radicals and anions immediately, and thus the trace amount of Ti ions might react with biomolecules, inducing body injury [13]. Indeed, the titanium release from hip-replacement components has resulted in titanium accumulation in serum and hair of patients with titanium alloy implants [24], probably because of the long-distance “travelled” by titanium [25]. Additionally, it has been suggested that the released titanium ions show high affinity for serum transferrin, binding the protein through metal binding sites [26].
There are many indications that titanium might cause some problems, e.g., “yellow nail syndrome” (YNS), allergic and autoimmune reactions. In view of this, it is possible and even necessary to discuss the toxicity and the allergy caused by titanium and its alloys [19]. Although numerous papers on titanium have been published, the chronic or sub-chronic effects on organs and various types of tissue, the dose-response correlations, and models of action have not been fully elucidated. Due to widespread use of titanium implants in prosthodontics and orthopedics, the most valuable data could be found in respective medical papers. It has been well known that titanium implants are in direct contact with body fluids (saliva) that contain various inorganic and organic compounds. In addition, the implant surfaces can be inhabited by bacteria, which might initiate the corrosion [27]. Although, titanium alloys are generally considered as passive under normal physiological conditions, some exceptions, such as oxide layer disruption or oral implant corrosion, have been reported [15][17]. For example, low pH, high concentration of fluoride (dental implants) and the presence of oxidizing agents are considered as the main factors inducing corrosion [28][29][30]. Moreover, it has been found that the toxicity of titanium alloys depends on the alloy composition [16][31][32][33][34][35], and thus the careful selection of the material should be performed [18]. The first-generation titanium alloys, which contain Cr, Ni, Be and Co, are very toxic, whereas those with Al and V exhibit little toxicity and slight allergic effects. On the other hand, new titania alloys containing Nb show favourable osteoconductive and osteoinductive properties due to the formation of an apatite layer on their surfaces, when exposed to an acidic environment [36]. Moreover, other cations (e.g., Ag, Cu, Zn and Ce) might present additional therapeutic effects, e.g., angiogenesis that is essential for cicatrize process, and antimicrobial properties [18][37]. According to Ikarashi et al. [38], titanium–zirconium (Ti–Zr) alloy-implants exhibit the best biocompatibility, improved properties (in respect to pure Ti) and a low level of fretting corrosion. Nowadays, toxicological effects, related to antibacterial properties of noble metals’ ions, such as Ag+ and Au+, which might be released by titanium alloys, have been a growing matter of concern [39]. Similarly, nanostructures/compounds containing antibiotics with the antibacterial, anti-infective and anti-inflammatory properties, have been under consideration. Although antibiotics are used to control invading organisms (mainly bacteria and protozoa) on the surface of implants, very often they cause some problems, including cell toxicity, allergic response, impairment of osteogenic activity and antibiotic-induced adverse reactions, e.g., superinfections and hypersensitivity [40][41][42].
The risk assessment of titanium and titanium alloys requires the quantification of unintended effects associated with a release of particular components. Obviously, this is not an easy task since very often contradictory data have been provided. For example, Rae [43] has postulated that pure titanium and titanium alloy (Ti-6Al-4V) do not affect human fibroblast cultures because of the relatively-low solubility of Ti ions [43]. By contrast, the corrosion products of titanium implants have been identified in serum and bone marrow, then being transported through the bloodstream and/or lymph to hair, lungs, spleen, liver and kidneys [44][45][46]. Accordingly, titanium has been detected in inner organs, including lungs, kidneys and liver, five months after a dental implant placement because of the translocation mechanisms [47]. Therefore, an estimation of changes in the content of Ti in the blood exposed to bone and dental implants, has been proposed as one of the toxicity indicators. Unfortunately, the changes of titanium content in the blood do not correlate with the implant-bone contact area, implants’ number and gender [48][49].
Other symptoms of implants’ corrosion include periprosthetic osteolysis, implant loosening and increased expression of proinflammatory mediators such as interleukins, prostaglandins, monocyte chemotactic proteins and macrophage colony stimulating factors [50][51][52][53]. Moreover, the particles of dental implants have been considered as initiators of destructive inflammatory processes, affecting tissues that surround dental implants—peri-implantitis [54][55][56]. For example, 100–300 ppm of titanium has been detected in trigger tissues [57]. The contact allergy to titanium might lead to pain, eczema, atopic dermatitis, swelling, erythema, urticaria and weakening of implants [15][57][58][59][60]. However, a difficulty in assessment of Ti allergy, because of uncertainty of the detection methods, seems to be the main problem [16]. In order to prevent implant failure, attention should be paid to a patient’s medical history to indicate the multiple allergies, e.g., to metals and jewellery [61].
A few studies indicate a possible connection between titanium and YNS [62][63][64]. The YNS or lymphedema associated with yellow nails is an uncommon and rare medical syndrome [63], characterized by slow nails’ growth, their yellow discoloration, lymphedema and tract involvement. In 2011, Berglund found a correlation between the titanium content in nail clippings and the yellowness and/or thickness of the nails. An excessive exposure to titanium from orthopedic implants along with ingestion of some drugs and foods (e.g., chewing gums, candies, chocolates) might be given as a probable cause of YNS. It seems extremely likely that the synergistic effect of chronic subthreshold is relevant. Moreover, fluoride-containing toothpastes and fluoride gels used for oral hygiene might exacerbate YSN. Berglund [62] has shown that titanium implants are a source of titanium ions, which are released from implants because of the galvanic action of dental gold and amalgam or oxidative reaction with fluorides [62]. Interestingly, the symptoms disappeared after stopping the galvanic reactions of titanium with other metals, and thus an exposure to titanium. Moreover, YNS might be dependent on underlying genetic and immunological disposition [63][64]. The experiments on animals have confirmed the titanium release from dental and orthopedic implants [27][56][65][66]. Furthermore, it has been found that the surface roughness of a metal insert is the most important factor of titanium release from the implant surface, i.e., the rougher the surface is, the higher is the coefficient of friction, and thus titanium release. In contrast, total area and diameter of implants are less important [66].
Titanium plasma-sprayed (TPS) implants should be considered as a special case, due to gradual and passive dissolution of their surface, which results in a decrease in the size of titanium particles with an increase of the distance from the implant surface. For example, titanium particles, released from TPS implants, have been detected at the average distance of 200–250 µm (till 500 µm) from the implants’ surface [67]. The analysis of histological sections has shown the presence of titanium at the distance from 0.4 mm up to 4.0 mm [27]. Generally, the Ti particles’ size, found in animal and human tissues, ranges from 10 nm to 54 µm [56]. It has been reported that Ti particles have a cytotoxicity effect through reduction of bone marrow stem cells (BMSCs) viability and proliferation, increase of p53 protein level, disruption of cell homeostasis and induction of DNA damage [68]. For example, Gomes et al. [69] showed (through an in vitro study) that Ti-6Al-4V alloy, widely used in medical and odonatological implants, presents a cytotoxic effect, i.e., the DNA damage (breaking of DNA strands) and mitotic spindle, leading to loss of whole chromosomes during cell division. However, the model of action is still unknown.
Two mechanisms of metal ions’ interactions with DNA have been considered: (1) direct and (2) indirect actions [70][71][72][73]. (1) Titanium as a transition metal (d-block metal) has incomplete d-orbital, and thus can bind directly to the DNA bases (N7 of purine or N3 of pyrimidine atom at G-C sites). On the other hand, (2) titanium has low-energy d orbitals, which suggests that indirect mechanism is more probable, i.e., based on increased formation of reactive oxygen species (ROS), and formation of hydrogen bonds between the coordinated ligands and negatively charged phosphate groups in DNA structure [38][70].
Considering the methods of toxicity evaluation, both Ti (alloy) particles and Ti ions have been investigated in vitro, ex vivo and in vivo, i.e., on DNA/RNA, protein, lipids, cells and animals. Accordingly, the cellular incorporation of titanium has been well studied, e.g., the cellular uptake efficiency is higher for titanium nanoparticles (NPs < 100 nm) than titanium microparticles (<44 μm). Moreover, only NPs have been observed in the nucleus, with 352 times higher cytotoxicity than microparticles [73]. However, it should be mentioned that large titanium particles could be incorporated into cells by phagocytosis [74]. Evans has evaluated the effect of titanium (mean size of 49 μm), ground titanium (14 μm) and titanium alloy (Ti90/Al6/V4, 8.9 μm) on the cell viability using two experimental conditions, i.e., (1) in the direct contact with cells, and (2) separated from them [75]. Although large titanium does not cause a decrease in the cell number under both conditions, small titanium significantly reduces the number of cells when they are in contact with titanium. Moreover, titanium alloy causes a higher reduction of cell number than ground titanium when in contact with cells. Accordingly, it has been proposed that small particles (5–10 μm) could induce cell damage by direct contact. The size-dependent cytotoxic effect of titanium particles/ions on neutrophils has also been shown, i.e., the fine titanium particles (1–3 μm) are incorporated into cells by phagocytosis causing the cytotoxicity [74][76], whereas Ti ions stimulate neutrophils and increase the quantity of released superoxide anions [74]. Moreover, it has been shown that the intraperitoneal injection of titania suspension induces the uptake of titanium by the blood cells (macrophages and phagocytic mononuclear cells) and its further dissemination to organs, such as liver, spleen and lungs via cells [44].
The cellular response to titanium particles/ions has been investigated mainly for oral mucosa cells. For example, it has been found that the exposure of mouse osteoblast-like MC3T3-E1 cells to Ti ions inhibits cell proliferation (in dependence on the concentration and time), and induces nuclear expression of Yes-associated protein YAP (a key transcription co-activator, the activity of which is inhibited by the Hippo signaling pathway) in osteoblasts, resulting in down regulation of osteogenic differentiation of MC3T3-E1 cells [77]. According to the in vivo study on detection of lactate dehydrogenase (LDH), interleukin (IL) and activated nuclear factor-kappa B (NF-κB), inflammatory reaction (high content of IL-6) and activated NF-κB have been detected around a titanium implant [78]. Moreover, it has been proposed that TNF-α, IL-1β, and IL-6 might induce osteoclastogenesis and inhibit osteoblastogenesis through the RANK–RANKL (receptor activator of nuclear factor kappa-Β–receptor activator of nuclear factor kappa-Β ligand) pathway [79]. Therefore, it has been concluded that titanium might induce inflammation. Moreover, it has been proposed that cells’ exposure to titanium might also influence the content of proteins and lipids. Indeed, titanium has caused a decrease in total protein content and some types of lipids, e.g., cholesterol ester and phosphatidylethanolamine, inducing the potential damage of tissues [80].
López-Jornet et al. [81] evaluated the DNA damage by dental implants in gingival cells, collected from patients with implants. The concentration of titanium (Ti47) in these cells was significantly higher than that in control cells (from patients without implants). The frequencies of micronuclei and binucleated cells, and nuclear buds in the “implant” group, have been higher than those in the control group, but without statistically significant differences. Moreover, during the study on the effect of Ti ions on osteoblast, Liao et al. [82] have revealed that the equal or higher concentration of Ti ions than 10 ppm inhibits cell proliferation. Additionally, it has been found that Ti ions: (i) reduce the expression of osteonectin and osteopontin mRNAs, (ii) delay the development of alkaline phosphatase mRNA expression, and (iii) decrease the enzyme activity.
It should be remembered that the toxic symptoms due to titanium are not only allergic reactions, but also disorders in a whole body. Fretwurst et al., (2016) have proposed that a release of Ti ions could participate in peri-implant bone loss [83]. Additionally, acidic conditions in an oral cavity might increase the corrosion of titanium [84]. Moreover, the induction of osteoclastogenesis and the inhibition of osteoblastogenesis can lead to bone resorption around joint replacements [79]. Mice treated with zirconium and titanium have expressed an inflammatory reaction and the reduction of bone surfaces in comparison to a sham group (PBS treated).
Despite the abundant content of titanium in the Earth’s crust, water contamination by abnormal content of titanium might also affect human health. Titanium has been found in river water, and thus accumulated in aquatic insects [85]. Therefore, its possible impacts on the food chain and the agricultural damage must be considered. Moreover, a statistical study in Mexico has suggested that titanium in the blood, ingested by insufficiently treated water, might be related to low haemoglobin content, and thus anaemia in children [86].
The effect of titanium on bacteria cells has also been investigated, but contradictory results have been reported, i.e., (i) no significant bactericidal effect on oral bacterial species [87][88], and (ii) bactericidal activity of titanium [89]. Recently, Stolzoff et al. [84] have revealed the effect of surface topography of titanium on bacteria. It has been found that a high density of uniformly sized nanofeatures prevents bacterial adhesion and proliferation [90]. Considering that bacteria might cause implant failure, therefore, the development of bacteria-resistant titania implants would be highly valuable for patients.
Summarizing, titanium is one of the safest metals, as it has been widely used for clinical implants. However, it is necessary to consider and evaluate carefully all possible negative impacts on human body. Moreover, clinicians should pay attention when titanium-based implants are installed in patients.
3. Toxicity of Titanium(IV) Oxide
Titania (titanium(IV) oxide, titanium dioxide, TiO2) is the most widely used titanium compound, and thus its toxicity should be carefully examined. Similar to titanium, titania has been reported as inert, and thus safe for humans and the environment for many years. Non-toxicity of titania has been listed as one of the main advantages of titania photocatalysts among high activity, chemical and thermal stability, broad availability and low costs. However, considering the nanoparticulate nature of titania photocatalysts, the nanosize-governed toxicity of titania has been postulated [91][92]. Accordingly, various studies on titania toxicity have been performed, as shown in Figure 2. More than 6000 papers have been published on “titania (titanium dioxide, TiO2) toxicity” (searched in Web of Science), and about 60% of them (3674 results) in the last five years. Accordingly, an evaluation of cytotoxic, ecotoxic, genotoxic and carcinogenic potential of TiO2 has been represented in large number of scientific papers (Figure 4). However, it should be pointed out that the possible toxicity of titania has been intensively studied only in the last few years, and some potential effects are still unknown, e.g., carcinogenicity (only ca. 10 papers/year), which might suggest the low toxic effect of titania. Accordingly, the possible hazardous impact of TiO2 has been reviewed and discussed in this review paper [https://doi.org/10.3390/nano10102065], including carcinogenicity.
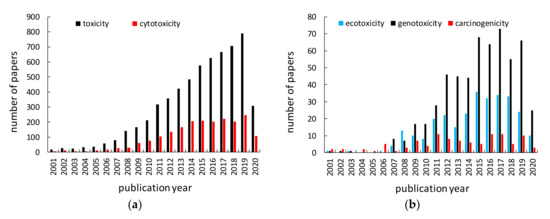
Figure 2. Number of papers published annually on titanium toxicity searched in Web of Science (May 25, 2020) using: left) “titanium dioxide toxicity” or “TiO2 toxicity” or “titania toxicity” (black) and “titanium dioxide cytotoxicity” or “TiO2 cytotoxicity” or “titania cytotoxicity” (red), right) “titanium dioxide ecotoxicity” or “TiO2 ecotoxicity” or “titania ecotoxicity” (blue), “titanium dioxide genotoxicity” or “TiO2 genotoxicity” or “titania genotoxicity” (black) and “titanium dioxide carcinogenicity” or “TiO2 carcinogenicity” or “titania carcinogenicity” (red).
4. Summary and Conclusions
The correct evaluation of the risk of titanium and its compounds requires understanding of all the factors involved in their behavior, as well as the generation of toxicity. As shown the effects of titanium and its compounds depend on the physicochemical properties, tested organisms, an exposure methodology (e.g., in vivo or in vitro, ex situ or in situ), an exposure time and thus full characterization of all factors must be considered when toxicity is discussed.
It might be concluded that there are not fully convincing studies titanium implants and titania photocatalysts cause serious health and environmental problems. However, broad applications of titanium compounds result in their accumulation in various organisms and environment, and thus disturbing environmental sustainability. Moreover, an excessive accumulation of titanium (as well as any other elements) possesses a threat, and thus cannot be ignored. Therefore, it should be carefully considered if the use of titanium and its compounds is necessary, reasonable, and causes more pros than cons.
It has been thought that the studies indicating titania toxicity (or the contact allergy) to human and animals cannot be omitted, and all people having the direct contact with titania need to be aware of sporadic problems of its noxiousness. The efforts should be made to obtain the scientifically sound toxicity data from the toxicity tests in the nearest future. It is believed that those data would result in avoidance of an unnecessary protection burden for industry (e.g., a need for Personal Protective Equipment PPE) and confusion for customers.
This entry is adapted from the peer-reviewed paper 10.3390/nano10102065
References
- Schwartz, M. Encyclopedia and Handbook of Materials, Parts and Finishes, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016.
- Hock, C.W. How TiCl3 Catalysts Control Texture of as-polymerized Polypropylene. Polym. Sci. 1966, 4, 3055–3065.
- Niinomi, M. Mechanical Properties of Biomedical Titanium Alloys. Mat. Sci. Eng. A Struct. 1998, 243, 231–236.
- Kawamura, Y.; Hayashi, K.; Inoue, A.; Masumoto, T. Rapidly Solidified Powder Metallurgy Mg97Zn1Y2 Alloys with Excellent Tensile Yield Strength above 600 MPa. Mater. Trans. 2001, 42, 1172–1176.
- Ezugwu, E.O. Key Improvements in the Machining of Difficult-to-cut Aerospace Superalloys. Int. J. Mach. Tools Manu. 2005, 45, 1353–1367.
- Ouyang, S.H.; Huang, Q.L.; Liu, Y.; Ouyang, Z.X.; Liang, L.X. Powder Metallurgical Ti-Mg Metal-metal Composites Facilitate Osteoconduction and Osseointegration for Orthopedic Application. Bioact. Mater. 2019, 4, 37–42.
- Xu, W.; Tian, J.; Liu, Z.; Lu, X.; Hayat, M.D.; Yan, Y.; Li, Z.; Qu, X.; Wen, C. Novel porous Ti35Zr28Nb scaffolds fabricated by powder metallurgy with excellent osteointegration ability for bone-tissue engineering applications. Mat. Sci. Eng. C Mater. 2019, 105, 110015.
- Maharubin, S.; Hu, Y.; Sooriyaarachchi, D.; Cong, W.; Tan, G.Z. Laser Engineered Net Shaping of Antimicrobial and Biocompatible Titanium-silver Alloys. Mat. Sci. Eng. C 2019, 105, 110059.
- Wu, H.; Xie, J.; Mao, L. One-pot assembly Tannic Acid-titanium Dual Network Coating for Low-pressure Nanofiltration Membranes. Sep. Purif. Technol. 2020, 233, 116051.
- Schkroeder, H.A.; Balassa, J.J.; Tipton, I.H. Abnormal Trace Metals in Man: Titanium. J. Chronic Dis. 1963, 16, 55–69.
- Branemark, P. Osseointegration and Its Experimental Background. J. Prosthet. Dent. 1983, 50, 399–410.
- Pouilleau, J.; Devilliers, D.; Garrido, F.; Durand-Vidal, S.; Mahé, E. Structure and Composition of Passive Titanium Oxide Films. Mat. Sci. Eng. B 1997, 47, 235–243.
- Hanawa, T. Metal Ion Release from Metal Implants. Mat. Sci. Eng. C 2004, 24, 745–752.
- Hanawa, T. Surface Treatment and Modification of Metals to Add Biofunction. Dent. Mater. J. 2017, 36, 533–538.
- Chaturvedi, T.P. An Overview of the Corrosion Aspect of Dental Implants (Titanium and its Alloys). Indian J. Dent. Res. 2009, 20, 91–98.
- Fage, S.W.; Muris, J.; Jakobsen, S.S.; Thyssen, J.P. Titanium: A Review on Exposure, Release, Penetration, Allergy, Epidemiology, and Clinical Reactivity. Contact Dermat. 2016, 74, 323–345.
- Zabrzyński, J.; Jaworski, Ł. Myths and Facts about Combining Metals in Orthopedic surgery. Chir. Narz. Ruchu Ortop. Pol. 2017, 82, 58–62.
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A Critical Review of Multifunctional Titanium Surfaces: New Frontiers for Improving Osseointegration and Host Response, Avoiding Bacteria Contamination. Acta Biomater. 2018, 79, 1–22.
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General Review of Titanium Toxicity. Int. J. Implant. Dent. 2019, 5, 10.
- Strietzel, R.; Hösch, A.; Kalbfleisch, H.; Buch, D. In Vitro Corrosion of Titanium. Biomaterials 1998, 19, 1495–1499.
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium Allergy in Dental Implant Patients: A Clinical Study on 1500 Consecutive Patients. Clin. Oral Implant. Res. 2008, 19, 823–835.
- Bhola, R.; Bhola, S.M.; Mishra, B.; Olson, D.L. Corrosion in Titanium Dental Implants/prostheses—A Review. Trends Biomater. Artif. Organs 2011, 25, 34–46.
- Evrard, L. Titanium: A new allergen. In Implant Dentistry—A Rapidly Evolving Practice; Turkyilmaz, I., Ed.; IntechOpen: London, UK, 2011.
- Jacobs, J.J.; Skipor, A.K.; Patterson, L.M.; Hallab, N.J.; Paprosky, W.G.; Black, J.; Galante, J.O. Metal Release in Patients Who Have Had a Primary Total Hip Arthroplasty: A Prospective, Controlled, Longitudinal Study. J. Bone Jt. Surg. 1998, 80, 1447–1458.
- Kasai, Y.; Iida, R.; Uchida, A. Metal Concentrations in the Serum and Hair of Patients with Titanium Alloy Spinal Implants. Spine 2003, 28, 1320–1326.
- Soto-Alvaredo, J.; Blanco, E.; Bettmer, J.; Hevia, D.; Sainz, R.M.; López Cháves, C.; Sánchez, C.; Llopis, J.; Sanz-Medel, A.; Montes-Bayón, M. Evaluation of the Biological Effect of Ti Generated Debris from Metal Implants: Ions and Nanoparticles. Metallomics 2014, 6, 1702–1708.
- Delgado-Ruiz, R.; Romanos, G. Potential Causes of Titanium Particle and Ion Release in Implant Dentistry: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 3585.
- Schiff, N.; Grosgogeat, B.; Lissac, M.; Dalard, F. Influence of Fluoride Content and pH on the Corrosion Resistance of Titanium and its Alloys. Biomaterials 2002, 23, 1995–2002.
- Yu, F.; Addison, O.; Davenport, A.J. A Synergistic Effect of Albumin and H2O2 Accelerates Corrosion of Ti6Al4V. Acta Biomater. 2015, 26, 355–365.
- Penarrieta-Juanito, G.; Sordi, M.B.; Henriques, B.; Dotto, M.E.R.; Teughels, W.; Silva, F.S.; Magini, R.S.; Souza, J.C.M. Surface Damage of Dental Implant Systems and Ions Release After Exposure to Fluoride and Hydrogen Peroxide. J. Periodontal. Res. 2019, 54, 46–52.
- Haynes, D.R.; Rogers, S.D.; Hay, S.; Pearcy, M.J.; Howie, D.W. The Differences in Toxicity and Release of Bone-resorbing Mediators Induced by Titanium and Cobalt-chromium-alloy Wear Particles. J. Bone Jt. Surg. 1993, 75, 825–834.
- Shettlemore, M.G.; Bundy, K.J. Toxicity Measurement of Orthopedic Implant Alloy Degradation Products Using a Bioluminescent Bacterial Assay. J. Biomed. Mater. Res. 1999, 45, 395–403.
- Chen, Q.; Thouas, G.A. Metallic Implant Biomaterials. Mat. Sci. Eng. R. 2015, 87, 1–57.
- Khadija, G.; Saleem, A.; Akhtara, Z.; Naqvi, Z.; Gull, M.; Masood, M.; Mukhtar, S.; Batool, M.; Saleem, N.; Rasheed, T.; et al. Short Exposure to Titanium, Aluminum and Vanadium (Ti 6Al 4V) Alloy Powder Drastically Affects Behavior and Antioxidant Metabolites in Vital Organs of Male Albino Mice. Toxicol. Rep. 2018, 5, 765–770.
- Kovan, V.; Tugce, T.; Topal, E.S. The Effect of Molybdenum on Titanium’s Castability in Dental Prosthesis Applications: A Numerical Analysis. Proc. Inst. Mech. Eng. 2019, L 233, 1966–1971.
- McMahon, R.E.; Ma, J.; Verkhoturov, S.V.; Munoz-Pinto, D.; Karaman, I.; Rubitschek, F.; Maier, H.J.; Hahn, M.S. A Comparative Study of the Cytotoxicity and Corrosion Nickel-Titanium and Titanium-niobium Shape Memory Alloys. Acta Biomater. 2012, 8, 2863–2870.
- Kirmanidou, T.; Sidira, M.; Drosou, M.E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michailidis, N.; Michalakis, K. New Ti-Alloys and Surface Modifications to Improve the Mechanical Properties and the Biological Response to Orthopedic and Dental Implants: A Review. BioMed Res. Int. 2016, 2, 1–21.
- Ikarashi, Y.; Toyoda, K.; Kobayashi, E.; Doi, H.; Yoneyama, T.; Hamanaka, H.; Tsuchiya, T. Improved Biocompatibility of Titanium-zirconium (Ti–Zr) Alloy: Tissue Reaction and Sensitization to Ti–Zr Alloy Compared with Pure Ti and Zr in Rat Implantation Study. Mater. Trans. 2005, 46, 2260–2226.
- Rehbock, C.; Jakobi, J.; Gamrad, L.; Van Der Meer, S.; Tiedemann, D.; Taylor, U.; Kues, W.; Rath, D.; Barcikowski, S. Current State of Laser Synthesis of Metal and Alloy Nanoparticles as Ligand-free Reference Materials for Nano-toxicological Assays. Beilstein J. Nanotechnol. 2014, 5,1523–1541.
- Lee, D.W.; Yun, Y.P.; Park, K.; Kim, S.E. Gentamicin and Bone Morphogenic Protein-2 (BMP-2)-delivering heparinized-titanium Implant with Enhanced Antibacterial Activity and Osteointegration. Bone 2012, 50, 974–982.
- Grischke, J.; Eberhard, J.; Stiesch, M. Antimicrobial Dental Implant Functionalization Strategies—A Systematic Review. Dent. Mat. J. 2016, 35, 545–558.
- Mandell, J.B.; Deslouches, B.; Montelaro, R.C.; Shanks, R.M.Q.; Doi, Y.; Urish, K.L. Elimination of Antibiotic Resistant Surgical Implant Biofilms Using an Engineered Cationic Amphipathic Peptide WLBU2. Sci. Rep. 2017, 7, 18098.
- Rae, T. The Toxicity of Metals Used in Orthopaedic Prostheses. An Experimental Study Using Cultured Human Synovial Fibroblasts. J. Bone Jt. Surg. 1981, 63-B, 435–440.
- Olmedo, D.G.; Tasat, D.; Guglielmotti, M.B.; Cabrini, R.L. Titanium Transport Through the Blood Stream. An Experimental Study on Rats. J. Mater. Sci. Mater. Med. 2013, 14, 1099–1103.
- He, X.; Reichl, F.X.; Wang, Y.; Michalke, B.; Milz, S.; Yang, Y.; Stolper, P.; Lindemaier, G.; Graw, M.; Hickel, R.; et al. Analysis of Titanium and Other Metals in Human Jawbones with Dental Implants—A Case Series Study. Dent. Mater. 2016, 32, 1042–1051.
- Lukina, E.; Laka, A.; Kollerov, M.; Sampiev, M.; Mason, P.; Wagstaff, P.; Noordeen, H.; Yoon, W.W.; Blunn, G. Metal Concentrations in the Blood and Tissues After Implantation of Titanium Growth Guidance Sliding Instrumentation. Spine J. 2016, 16, 380–388.
- Schliephake, H.; Reiss, G.; Urban, R.; Neukam, F.W.; Guckel, S. Metal Release from Titanium Fixtures During Placement in the Mandible: An Experimental Study. Int. J. Oral Maxillofac. Implant. 1993, 8, 502–511.
- Ipach, I.; Schäfer, R.; Mittag, F.; Leichtle, C.; Wolf, P.; Kluba, T. The Development of Whole Blood Titanium Levels After Instrumented Spinal Fusion—Is There a Correlation Between the Number of Fused Segments and Titanium Levels? BMC Musculoskelet. Disord 2012, 13, 159.
- Temiz, M.; Dayi, E.; Saruhan, N. Evaluation of Blood Titanium Levels and Total Bone Contact Area of Dental Implants. BioMed Res. Int. 2018, 4121639.
- Warme, B.A.; Epstein, N.J.; Trindade, M.C.; Miyanishi, K.; Ma, T.; Saket, R.R.; Regula, D.; Goodman, S.B.; Smith, R.L. Proinflammatory Mediator Expression in a Novel Murine Model of Titanium-particle-induced Intramedullary Inflammation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 71, 360–366.
- Dalal, A.; Pawar, V.; McAllister, K.; Weaver, C.; Hallab, N.J. Orthopedic Implant Cobalt-alloy Particles Produce Greater Toxicity and Inflammatory Cytokines than Titanium Alloy and Zirconium Alloy-based Particles in vitro, in Human Osteoblasts, Fibroblasts, and Macrophages. J. Biomed. Mater. Res. Part A 2012, 100, 2147–2158.
- Wachi, T.; Shuto, T.; Shinohara, Y.; Matono, Y.; Makihira, S. Release of Titanium Ions from an Implant Surface and their Effect on Cytokine Production Related to Alveolar Bone Resorption. Toxicology 2015, 327, 1–9.
- Safioti, L.M.; Kotsakis, G.A.; Pozhitkov, A.E.; Chung, W.O.; Daubert, D.M. Increased Levels of Dissolved Titanium are Associated with Peri-implantitis—A Cross-sectional Study. J. Periodontol. 2017, 88, 436–442.
- Hatami, M.; Ghorbanpour, M.; Salehiarjomand, H. Nano-anatase TiO2 Modulates the Germination Behavior and Seedling Vigority of Some Commercially Important Medicinal and Aromatic Plants. J. Biol. Environ. Sci. 2014, 8, 53–59.
- Penmetsa, S.L.D.; Shah, R.; Thomas, R.; Kumar, A.B.T.; Gayatri, P.S.D.; Mehta, D.S. Titanium particles in tissues from peri-implant mucositis: An exfoliative cytology-based pilot study. J. Indian Soc. Periodontol. 2017, 21, 192–194.
- Suárez-López del Amo, F.; Garaicoa-Pazmiño, C.; Fretwurst, T.; Castilho, R.M.; Squarize, C.H. Dental Implants—Associated release of Titanium Particles: A A systematic Review. Clin. Oral Implant. Res. 2018, 29, 1085–1100.
- Goutam, M.; Giriyapura, C.; Mishra, S.K.; Gupta, S. Titanium Allergy: A Literature Review. Indian J. Dermatol. 2014, 59, 630.
- Bircher, A.J.; Stern, W.B. Allergic Contact Dermatitis from “Titanium” Spectacle Frames. Contact Dermat. 2001, 45, 244–245.
- Egusa, H.; Ko, N.; Shimazu, T.; Yatani, H. Suspected Association of An Allergic Reaction with Titanium Dental Implants: A Clinical Report. J. Prosthet. Dent. 2008, 100, 344–347.
- Thomas, P.; Bandl, W.D.; Maier, S.; Summer, B.; Przybilla, B. Hypersensitivity to Titanium Osteosynthesis with Impaired Fracture Healing, Eczema, and T-cell Hyperresponsiveness In Vitro: Case Report and Review of the Literature. Contact Dermat. 2006, 55, 199–202.
- Wood, W.; Vermilyea, S.G. A Review of Selected Dental Literature on Evidence-based Treatment Planning for Dental Implants: Reports of the Committee on Research in Fixed Prosthodontics of the Academy of Fixed Prosthodontics. J. Prosthet. Dent. 2004, 92, 447–462.
- Berglund, F. Titanium and Yellow Nail Syndrome. In Novel Strategies in Lymphedema; EPublishing; Vannelli, A., Ed.; IntechOpen: London, UK, 2011.
- Decker, A.; Daly, D.; Scher, R.K. Role of Titanium in the Development of Yellow Nail Syndrome. Skin Appendage Disord. 2015, 1, 28–30.
- Ataya, A.; Kline, K.P.; Cope, J.; Alnuaimat, H. Titanium Exposure and Yellow Nail Syndrome. Respir. Med. Case Rep. 2015, 16, 146–147.
- Bianco, P.D.; Ducheyne, P.; Cuckler, J.M. Local Accumulation of Titanium Released from a Titanium Implant in the Absence of Wear. J. Biomed. Mater. Res. 1996, 31, 227–234.
- Pettersson, M.; Pettersson, J.; Thoren, M.M.; Johansson, A. Release of Titanium After Insertion of Dental Implants with Different Surface Characteristics—An ex vivo Animal Study. Acta Biomater. Odontol. Scand. 2018, 3, 63–73.
- Martini, D.; Fini, M.; Franchi, M.; Pasquale, V.D.; Bacchelli, B.; Gamberini, M.; Tinti, A.; Taddei, P.; Giavaresi, G.; Ottani, V.; et al. Detachment of Titanium and Fluorohydroxyapatite Particles in Unloaded Endosseous Implants. Biomaterials 2003, 24, 1309–1316.
- Meng, B.; Chen, J.; Guo, D.; Ye, Q.; Liang, X. The Effect of Titanium Particles on Rat Bone Marrow Stem Cells In Vitro. Toxicol. Mech. Method. 2009, 19, 552–558.
- Gomes, C.C.; Moreira, L.M.; Santos, V.J.S.V.; Ramos, A.S.; Lyon, J.P.; Soares, C.P.; Santos, F.V. Assessment of the Genetic Risks of a Metallic Alloy Used in Medical Implants. Genet. Mol. Biol. 2011, 34, 116–121.
- Anastassopoulou, J. Metal-DNA Interactions. J. Mol. Struct. 2003, 651-653, 19–23.
- Shamsi, M.H.; Kraatz, H.M. Interactions of Metal Ions with DNA and Some Applications. J. Inorg. Organomet. Polym. 2012, 23, 4–23.
- Egorova, K.S.; Ananikov, V.P. Toxicity of Metal Compounds: Knowledge and Myths. Organometallics 2017, 36, 4071–4090.
- He, X.; Hartlieb, E.; Rothmund, L.; Waschke, J.; Wu, X.; Van Landuyt, K.L.; Milz, S.; Michalke, B.; Hickel, R.; Reichl, F.X.; et al. Intracellular Uptake and Toxicity of Three Different Titanium Particle. Dental Mater. 2015, 31, 734–744.
- Kumazawa, R.; Watari, F.; Takashi, N.; Tanimura, Y.; Uo, M.; Totsuka, Y. Effects of Ti Ions and Particles on Neutrophil Function and Morphology. Biomaterials 2002, 23, 3757–3764.
- Evans, E.J. Cell Damage In Vitro Following Direct Contact with Fine Particles of Titanium, Titanium Alloy and Cobalt-chrome-molybdenum Alloy. Biomaterials 1994, 15, 713–717.
- Tamura, K.; Takashi, N.; Kumazawa, R.; Watari, F.; Totsuka, Y. Effects of Particle Size on Cell Function and Morphology in Titanium and Nickel. Mater. Trans. 2012, 43, 3052–3057.
- Zhu, W.; Ming, P.; Qiu, J.; Shao, S.; Yu, Y.; Chen, J.; Yang, J.; Xu, L.; Zhang, S.; Tang, C. Effect of Titanium Ions on the Hippo/YAP Signaling Pathway in Regulating Biological Behaviors of MC3T3-E1 Osteoblasts. J. Appl. Toxicol. 2018, 38, 824–833.
- Suska, F.; Gretzer, C.; Esposito, M.; Emanuelsson, L.; Wennerberg, A.; Tengvall, P.; Thomsen, P. In Vivo Cytokine Secretion and NF-κB Activation Around Titanium and Copper Implants. Biomaterials 2005, 26, 519–527.
- Obando-Pereda, G.A.; Fischer, L.; Stach-Machado, D.R. Titanium and Zirconia Particle-induced Pro-inflammatory Gene Expression in Cultured Macrophages and Osteolysis, Inflammatory Hyperalgesia and Edema In Vivo. Life Sci. 2014, 97, 96–106.
- Schuster, G.S.; Caughman, G.B. Alterations of Cell Lipids by Metal Salts. J. Biomed. Mater. Res. A 2004, 70, 347–353.
- López-Jornet, P.; Perrez, F.P.; Calvo-Guirado, J.L.; Lor-Ros, I.L.; Ramírez-Fernández, P. Metallic Ion Content and Damage to the DNA in Oral Mucosa Cells Patients Treated Dental Implants. J. Mater. Sci. Mater. Med. 2014, 25, 1819–1824.
- Liao, H.; Wurtz, T.; Li, J. Influence of Titanium Ion on Mineral Formation and Properties of Osteoid Nodules in Rat Calvaria Cultures. J. Biomed. Mater. Res. 1999, 47, 220–227.
- Fretwurst, T.; Buzanich, G.; Nahles, S.; Woelber, J.P.; Riesemeier, H.; Nelson, K. Metal Elements in Tissue with Dental Peri-implantitis: A Pilot Study. Clin. Oral Implants Res. 2016, 27, 1178–1186.
- Fretwurst, T.; Nelson, K.; Tarnow, D.P.; Wang, H.L.; Giannobile, W.V. Is Metal Particle Release Associated with Peri-implant Bone Destruction? An Emerging Concept. J. Dent. Res. 2018, 97, 259–265.
- Aydoğan, Z.; Sisman, T.; Icekara, U.; Gurol, A. Heavy Metal Accumulation in Some Aquatic Insects (Coleoptera: Hydrophilidae) and Tissue of Chondrostoma regium (Heckel, 1843) Relevant to Their Concentration in Water and Sediments from Karasu River, Erzurum, Turkey. Environ. Sci. Pollut. Res. 2017, 24, 9566–9574.
- López-Rodríguez, G.; Galván, M.; González-Unzaga, M.; Ávila, J.H.; Pérez-Labra, M. Blood Toxic Metals and Hemoglobin Levels in Mexican Children. Environ. Monit. Assess. 2017, 189, 179.
- Elagli, K.; Neut, C.; Romond, C.; Hildebrand, H.F. In Vitro Effects of Titanium Powder on Oral Bacteria. Biomaterials 1992, 13, 25–27.
- Leonhardt, A.; Dahlén, G. Effect of Titanium on Selected Oral Bacterial Species in Vitro. Eur. J. Oral Sci. 1995, 103, 382–387.
- Berry, C.W.; Moore, T.J.; Safar, J.A.; Henry, C.A.; Wagner, M.J. Antibacterial Activity of Dental Implant Metals. Implant Dent. 1992, 1, 59–65.
- Stolzoff, M.; Burns, J.E.; Aslani, A.; Tobin, E.J.; Nguyen, C.; De La Torre, N.; Golshan, N.H.; Ziemer, K.S.; Webster, T.J. Decreased Bacterial Growth on Titanium Nanoscale Topographies Created by Ion Beam Assisted Evaporation. Int. J. Nanomed. 2017, 12, 1161–1169.
- Pichat, P. A Brief Survey of the Potential Health Risks of TiO2 Particles and TiO2-Containing Photocatalytic and Non-photocatalytic Materials. J. Adv. Oxid. Technol. 2010, 13, 238–246.
- Friehs, E.; Al Salka, Y.; Jonczyk, R.; Lavrentieva, A.; Jochums, A.; Walter, J.G.; Stahl, F.; Scheper, T.; Bahnemann, D. Toxicity, Phototoxicity and Biocidal Activity of Nanoparticles Employed in Photocatalysis. J. Photochem. Photobiol. C 2016, 29, 1–28.
