The SARS-CoV-2 spike protein, a class I viral fusion protein, is critical to initiating the interactions between the virus and the host cell surface receptor, facilitating viral entry into the host cell by assisting in the fusion of the viral and host cell membranes. Human host cells also sensitively respond to the spike protein to elicit cell signaling.
- cell signaling
- coronavirus
- COVID-19
- SARS-CoV-2
- spike protein
- vaccine
1. Introduction
The world is suffering from the coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a positive-sense, single-stranded RNA virus [1][2]. As of the end of December 2020, over 80 million people have been infected with SARS-CoV-2, causing 1.8 million deaths worldwide. SARS-CoV-2 uses its viral membrane fusion protein, known as a spike protein, to bind to angiotensin converting enzyme 2 (ACE2) as a ‘receptor’ in order to enter human host cells [3][4], causing severe pneumonia and acute respiratory distress syndrome (ARDS) [5]. Elderly patients with cardiovascular disease are particularly susceptible to developing serious COVID-19 conditions that in some cases lead to death, while young and healthy individuals are largely resistant to developing severe symptoms [1][6][7]. As COVID-19 continues to cause serious health, economic, and sociological problems, the world awaits the widespread rollout of effective vaccines that may end this pandemic.
The SARS-CoV-2 spike protein, a class I viral fusion protein, is critical to initiating the interactions between the virus and the host cell surface receptor, facilitating viral entry into the host cell by assisting in the fusion of the viral and host cell membranes. This protein consists of two subunits: Subunit 1 (S1) that contains the ACE2 receptor-binding domain (RBD) and Subunit 2 (S2) that plays a role in the fusion process [3][4] (Figure 1). The SARS-CoV-2 spike protein is the major target for the development of COVID-19 vaccines.
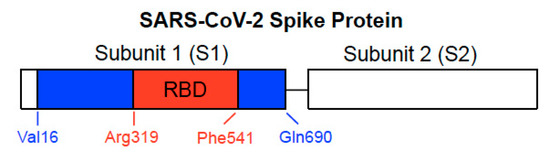
Figure 1. Structure of SARS-CoV-2 spike protein. The spike protein consists of Subunit 1 (S1) and Subunit 2 (S2). The S1 subunit contains the receptor-binding domain (RBD) that binds to ACE2 of the host cell membrane. The S2 subunit is responsible for fusion. In our previous study, we used full-length S1 (Val16-Gln690) depicted with blue and red regions and the RBD only-containing protein (Arg319-Phe541) shown in red of the SARS-CoV-2 spike protein (GenBank Accession Number: QHD43416.1).
2. Development of Spike Protein-Based COVID-19 Vaccines
The remarkably rapid development of vaccines and therapeutics for COVID-19 in 2020 has been due to effective collaborations between governments and the private sector. On 9 November 2020, Pfizer and BioNTech announced that their mRNA-based vaccine candidate, BNT162b2, is more than 90% effective against COVID-19 [8]. This was welcome news in that it revealed that effective vaccines may soon become available. BNT162b2 encodes the SARS-CoV-2 spike protein to elicit virus-neutralizing antibodies [9][10]. More specifically, it encodes the full-length spike protein of SARS-CoV-2 with two amino acids mutated to proline in the S2 subunit to maintain the prefusion conformation, while its sister vaccine BNT162b1 (also from Pfizer/BioNTech) encodes only the RBD of the SARS-CoV-2 spike protein, trimerized by the addition of a T4 fibritin foldon domain [9][10][11]. Clinical trials have demonstrated that neither BNT162b1 [11] nor BNT162b12 [9][10] exhibit serious short-term adverse effects. On 10 December 2020, the results of a large clinical trial for BNT162b were published, showing that this vaccine conferred 95% protection in persons 16 years of age or older [12]. Long-term consequences of these vaccines are, however, unknown.
Another promising vaccine, mRNA-1273 by Moderna, is also an RNA vaccine that encodes the full-length SARS-CoV-2 spike protein [13]. Viral vector-based vaccines such as AZD1222 by AstraZeneca, which uses a non-replicating chimpanzee adenovirus vector [14], Ad26.COV2.S by Johnson & Johnson, a non-replicating adenovirus 26-based system [15], and Gam-COVID-Vac (Sputnik V) by Gamaleya Research Institute of Epidemiology and Microbiology [16], all express the SARS-CoV-2 spike protein. NVX-CoV2373 (Novavax), a recombinant protein-based vaccine [17], is also the full-length SARS-CoV-2 spike protein. These vaccines as well as many others under development [18][19][20] introduce the SARS-CoV-2 spike protein into our body, so that the production of antibodies and immunity against SARS-CoV-2 are stimulated.
3. SARS-CoV-2 Spike Protein Elicits Cell Signaling in Human Cells
It was found that the treatment of cultured primary human pulmonary artery smooth muscle cells (SMCs) or human pulmonary artery endothelial cells with the recombinant SARS-CoV-2 spike protein S1 subunit is sufficient to promote cell signaling without the rest of the viral components [21]. Furthermore, our analysis of the postmortem lung tissues of patients who died of COVID-19 has determined that these patients exhibited pulmonary vascular wall thickening, a hallmark of pulmonary arterial hypertension (PAH) [21]. Based on these results, we proposed that the SARS-CoV-2 spike protein (without the rest of the viral components) triggers cell signaling events that may promote pulmonary vascular remodeling and PAH as well as possibly other cardiovascular complications [21][22].
In our cell culture experiments, two recombinant SARS-CoV-2 spike proteins, both of which contain the RBD, were studied [21]. The full-length S1 subunit protein contains most of the S1 subunit (Val16–Gln690), while the RBD S1 subunit protein only contains the RBD region (Arg319–Phe541), as shown in Figure 1. Cultured primary human pulmonary artery SMCs and human pulmonary artery endothelial cells were treated with these proteins for 10 min. We found, using the phospho-specific MEK antibody, that the recombinant full-length S1 subunit of SARS-CoV-2 alone at a concentration as low as 130 pM activated MEK, the activator of extracellular signal-regulated kinase (ERK) and a well-known signal transduction mechanism for cell growth [23]. By contrast, such activation of cell signaling by the spike protein did not occur in rat pulmonary artery SMCs [21].
While ACE2 is now well known as a ‘receptor’ to which the SARS-CoV-2 spike protein binds on human host cells in order to facilitate the membrane fusion and gain viral entry, the usual physiological function of ACE2 is not to serve as a membrane receptor to transduce intracellular signals. ACE2 is a type I integral membrane protein that functions as a carboxypeptidase, cleaving angiotensin II to angiotensin (1–7) and regulating blood pressure [24][25] (Figure 2). However, ten years ago, Chen et al. [26] reported the intriguing findings showing that ACE2 acts as a membrane receptor for cell signal transduction in response to the spike protein of SARS-CoV (now also known as SARS-CoV-1, the virus that caused the SARS outbreak in 2002–2004) in the human lung alveolar epithelial cell line, A549. The spike protein of SARS-CoV-1 is 76–78% identical to that of SARS-CoV-2 [27]. In their study, it was shown that the binding of the full-length spike protein to ACE2 triggered the casein kinase II-dependent activation of activator protein-1 (AP-1) transcription factor and subsequent gene transcriptional events [26]. Their finding on SARS-CoV-1 [26] and ours on SARS-CoV-2 [21] indicate that the spike protein remarkably functionally converts ACE2 (that is normally a peptidase enzyme) into a membrane receptor for cell signaling that uses the spike protein as a ligand for its activation (Figure 2).
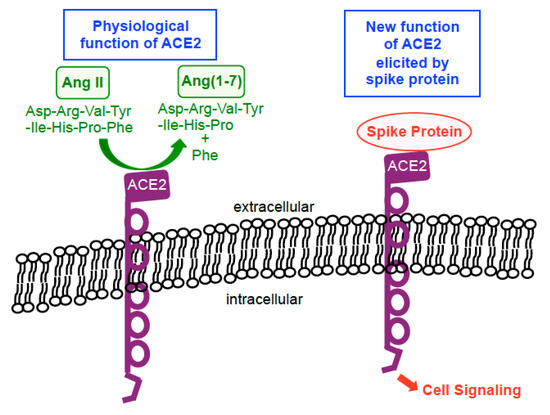
Figure 2. Biological functions of ACE2. In physiological situations, ACE2 functions as a carboxypeptidase enzyme that catalyzes the hydrolysis of angiotensin II (Ang II) into Ang(1–7) by cleaving off a phenylalanine (Phe). In the presence of the spike protein, this enzyme becomes a membrane receptor for cell signaling that uses the spike protein as a ligand for its activation.
Kuba et al. [28] showed that the injection of mice with recombinant SARS-CoV-1 spike protein reduced the ACE2 expression and worsened the acid-induced lung injury. In mice with an acid-induced lung injury, the recombinant SARS-CoV-1 spike protein dramatically increased angiotensin II, and the angiotensin receptor inhibitor losartan attenuated the spike protein-induced enhancement of lung injury [28]. Thus, these in vivo studies demonstrated that the spike protein of SARS-CoV-1 (without the rest of the virus) reduces the ACE2 expression, increases the level of angiotensin II, and exacerbates the lung injury.
The SARS-CoV-2 spike protein without the rest of the viral components has also been shown to activate cell signaling by Patra et al. [29]. The authors reported that the full-length SARS-CoV-2 spike protein expressed by the means of transient transfection, either in the human lung alveolar epithelial cell line A549 or in the human liver epithelial cell line Huh7.5, activated NF-κB and AP-1 transcription factors as well as p38 and ERK mitogen-activated protein kinases, releasing interleukin-6. This cell signaling cascade was found to be triggered by the SARS-CoV-2 spike protein downregulating the ACE2 protein expression, subsequently activating the angiotensin II type 1 receptor [29]. These experiments using transient transfection may reflect the intracellular effects of the spike protein that could be triggered by the RNA- and viral vector-based vaccines.
These results collectively reinforce the idea that human cells are sensitively affected by the extracellular and/or intracellular spike proteins though the activation of cell signal transduction.
4. RBD Only-Containing SARS-CoV-2 Spike Protein Does Not Elicit Cell Signaling in Human Cells
In contrast to the full-length spike protein [26][29] or the full-length SARS-CoV-2 spike protein S1 subunit [21], we found that the RBD only-containing protein (Figure 1) did not promote cell signaling. Our Western blotting results monitoring the MEK activation showed that the mean ± SEM phosphorylated MEK to MEK protein ratio values were 0.05 ± 0.003 (untreated), 1.9 ± 0.07 (treated with the full-length S1 protein), and 0.05 ± 0.003 (treated with the RBD only-containing protein) for human pulmonary artery SMCs; and 0.09 ± 0.006 (untreated), 0.90 ± 0.06 (treated with the full-length S1 protein), and 0.10 ± 0.003 (treated with the RBD only-containing protein) for human pulmonary artery endothelial cells [21].
The different effects of the full-length S1 and RBD only-containing proteins may be important considering that BNT162b2 and many other COVID-19 vaccines express the full-length spike protein, while the BNT162b1 vaccine encodes only the RBD region [9][10][11][12][13][14][15][16][17][18][19][20]. There are some other RBD-based COVID-19 vaccines being developed as well [30]. It is possible that the RBD-based vaccines are less immunogenic, but may not affect the host cells. Thus, they may be less risky considering potential long-term adverse effects. However, in the in vivo study of the SARS-CoV-1 spike protein described above [28], a deletion mutant that only contained the RBD also worsened the acid-induced lung failure, like the full-length spike protein. Thus, further work is needed to understand effects of the full-length spike protein and the RBD-only containing protein in various biological processes.
This entry is adapted from the peer-reviewed paper 10.3390/vaccines9010036
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients in-fected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020, e200994.
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE. Science 2020, 367, 1444–1448.
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620.
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422.
- Li, B.; Yang, J.; Zhao, F.; Zhi, L.; Wang, X.; Liu, L.; Bi, Z.; Zhao, Y. Prevalence and impact of cardiovascular metabolic diseas-es on COVID-19 in China. Clin. Res. Cardiol. 2020, 109, 531–538.
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95.
- Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study. Available online: https://www.businesswire.com/news/home/20201109005539/en/ (accessed on 9 November 2020).
- Walsh, E.E.; Frenck, R.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. RNA-based COVID-19 vaccine BNT162b2 selected for a pivotal efficacy study. medRxiv 2020, doi:10.1101/2020.08.17.20176651v2.
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N. Engl. J. Med. 2020, 383, 2439–2450.
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593.
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615, doi:10.1056/NEJMoa2034577. (in press)
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931.
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clut-terbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478.
- Mercado, N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; McMahan, K.; Tostanoski, L.H.; et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020, 586, 583–588.
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897.
- Guebre-Xabier, M.; Patel, N.; Tian, J.-H.; Zhou, B.; Maciejewski, S.; Lam, K.; Portnoff, A.D.; Massare, M.J.; Frieman, M.B.; Piedra, P.A.; et al. NVX-CoV2373 vaccine protects cynomolgus macaque upper and lower airways against SARS-CoV-2 challenge. Vaccine 2020, 38, 7892–7896.
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020, 288, 198114.
- Dong, Y.; Dai, T.; Wei, Y.; Zhang, L.; Zheng, M.; Zhou, F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct. Target. Ther. 2020, 5, 237.
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527.
- Suzuki, Y.J.; Nikolaienko, S.I.; Dibrova, V.A.; Dibrova, Y.V.; Vasylyk, V.M.; Novikov, M.Y.; Shults, N.V.; Gychka, S.G. SARS-CoV-2 spike protein-mediated cell signaling in lung vascular cells. Vascul. Pharmacol. 2020, 106823, doi:10.1016/j.vph.2020.106823. (Online ahead of Print)
- Suzuki, Y.J. The viral protein fragment theory of COVID-19 pathogenesis. Med. Hypotheses 2020, 144, 110267.
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18.
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angioten-sin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniver-sary of the discovery of ACE. Circ. Res. 2020, 126, 1456–1474.
- Warner, F.J.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Angiotensin-converting enzyme-2: A molecular and cellular perspective. Cell. Mol. Life Sci. 2004, 61, 2704–2713.
- Chen, P.I.; Chang, S.C.; Wu, H.Y.; Yu, T.C.; Wei, W.C.; Lin, S.; Chien, C.L.; Chang, M.F. Upregulation of the chemokine (C-C motif) ligand 2 via a severe acute respiratory syndrome coronavirus spike-ACE2 signaling. J. Virol. 2010, 84, 7703–7712.
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020, 94, e00127–e00120.
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angio-tensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005, 11, 875–879.
- Patra, T.; Meyer, K.; Geerling, L.; Isbell, T.S.; Hoft, D.F.: Brien, J.; Pinto, A.K.; Ray, R.B.; Ray, R. SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells. PLoS Pathog. 2020, 16, e1009128.
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2020; 1-10. doi: 10.1038/s41577-020-00480-0. Online ahead of print.
