Dissociated optic nerve fiber layer (DONFL) appearance is characterized by dimpling of the fundus when observed after vitrectomy with the internal limiting membrane (ILM) peeling in macular diseases. However, the cause of DONFL remains largely unknown. Optical coherence tomography (OCT) findings have indicated that the nerve fiber layer (NFL) and ganglion cells are likely to have been damaged in patients with DONFL appearance. Since DONFL appearance occurs at a certain postoperative period, it is unlikely to be retinal damage directly caused by ILM peeling because apoptosis occurs at a certain period after tissue damage and/or injury. However, it may be due to ILM peeling-induced apoptosis in the retinal tissue. Anoikis is a type of apoptosis that occurs in anchorage-dependent cells upon detachment of those cells from the surrounding extracellular matrix (i.e., the loss of cell anchorage). The anoikis-related proteins βA3/A1 crystallin and E-cadherin are reportedly expressed in retinal ganglion cells.
- macular hole (MH)
- vitrectomy
- internal limiting membrane (ILM)
- dissociated optic nerve fiber layer (DONFL)
- optical coherence tomography (OCT)
- anoikis
- βA3/A1 crystallin
- E-cadherin
- retinal ganglion cell
1. Introduction
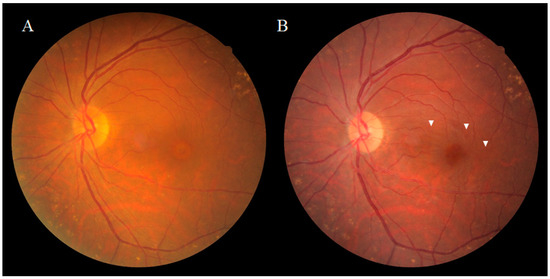
Dissociated optic nerve fiber layer (DONFL) appearance, a fundus finding first reported by Tadayoni et al. [1] in 2001, is observed after vitrectomy with the internal limiting membrane (ILM) peeling in macular diseases, such as a macular hole (MH) and the epiretinal membrane (ERM), or for rhegmatogenous retinal detachment [2,3]. It has also been reported that the appearance of DONFL can occur after secondary ILM peeling for hematomas under the ILM [4]. Ophthalmoscopically, DONFL appearance features arcuate striae color changes, which appear to be multilinear in shape, that are frequently observed in the macular nerve fiber layer (NFL) (Figure 1A,B). Since the appearance of DONFL is not common in patients who have undergone vitrectomy without ILM peeling [2,3], it is thought that ILM peeling is certainly involved in its occurrence. Reportedly, DONFL appearance commonly occurs at 1–3 months after vitrectomy, and thereafter, persists in some patients or becomes slightly less distinct in other patients [5,6]. Although the incidence of DONFL varies from report to report, it apparently occurs in approximately 30–60% of MHs in which an ILM peel is performed [1,2,3,4,5,6]. Although previous studies have suggested various factors associated with DONFL appearance, its exact cause remains unclear.
2. Anatomical Features of DONFL Appearance
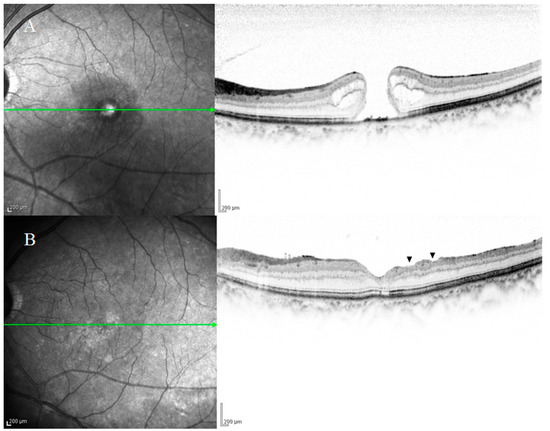
While DONFL appearance can be observed by ophthalmoscopy or color fundus photography, more detailed graphic images can be obtained by infrared fundus photography or optical coherence tomography (OCT). Kishimoto et al. [6] reported that examination by OCT is highly likely to detect DONFL appearance even when it cannot be detected on color fundus photographs. Kusuhara et al. [7] and Pichi et al. [8] reported that “en face” OCT layer imaging was useful for the diagnosis of DONFL appearance. Reportedly, when scanned vertically to the arcuate striae of DONFL appearance using OCT, small dimples are observed in the retinal NFL (RNFL), which are consistent with the striae [9]. In patients without DONFL appearance, OCT fails to visualize distinct dimples in the RNFL. In a report by Miura et al. [10], the authors demonstrated using scanning laser ophthalmoscopy (SLO) of an MH that DONFL appearance is a change in the retinal surface. In a report by Mitamura et al. [9], the authors measured the RNFL thickness (i.e., the mean RNFL thickness at both ends of the dimple site) and the depth of the dimples at three sites in patients using OCT, and their findings showed that the mean RNFL thickness at the dimple margins was 38.1 ± 9.3 μm, that the depth of each dimple was 28.6 ± 8.0 μm, and that the depths of all dimples measured were limited to the RNFL thickness. Kim et al. [11] reported that DONFL appearance gradually increased after surgery and that in 57.7% of the cases, DONFL appearance was confined to the NFL at 6-months postoperative, while in 30.8% of the cases, DONFL appearance extended to the inner plexiform layer. It should be noted that Demirel et al. [12] also reported results consistent with those findings.
2.1. DONFL Appearance and Visual Function
2.2. Previous Studies on the Pathogenesis of DONFL Appearance
Since the ILM is the basement membrane of Müller cells, ILM peeling during vitreous surgery may damage Müller cells. Runkle et al. [13] assessed the relationship between DONFL and intraoperative membrane-peeling dynamics as visualized using intraoperative OCT and evaluated the functional implications of DONFL. Their findings showed the acute post-peel increase in inner retinal thickness, and they concluded that one mechanism in the development of DONFL appearance may be intraoperative trauma to the inner retina, potentially during ILM peeling. In regard to this matter, Tadayoni et al. [1] proposed two hypotheses, the first being that the inner surface of the ILM is smooth, but its outer surface is irregular, and thus, DONFL appearance is an irregularity on the retinal surface after ILM peeling, and the second being that DONFL appearance is caused by the cleavage of the nerve fiber bundles due to ILM peeling-associated damage to the Müller cells, which keep the optic nerve fiber bundles close together. Steel et al. [16] described that the extent of DONFL observed postoperatively can be partly explained by the amount of cellular debris on the retinal side of the peeled ILM, while Park et al. [17] reportedly found no difference in the frequency of DONFL on the use of indocyanine green. Kim et al. [11] assessed the association between the appearance of DONFL after ILM peeling and changes in the macular NFL area by spectral-domain OCT. Their findings showed that there was no significant difference in average macular NFL area between group I (presence of DONFL) and group II (absence of DONFL) at 6-months postoperative. Based on these results, they reported that DONFL appearance started to occur at approximately 1-month postoperatively, and was thus unlikely to be mechanical damage to the NFL caused by ILM peeling and that DONFL appearance might represent macular NFL rearrangement and reorganization [5]. Spaide [18] reported that the correlation of the volume-rendered images with B-scan spectral-domain OCT showed focal areas of thinning of the ganglion cell layer with decreased reflectivity from the NFL in the areas of DONFL. They also described that DONFL seems to be the result of an interplay between trauma and healing processes constrained by NFL and does not appear to be due to the dissociation of optic nerve fibers. As Spaide noted, the word “dissociated” may not be correct, and the term “inner retinal dimpling” may be more appropriate. Their findings also reportedly showed that DONFL appearance is the result of an interplay between the damage to Müller cells caused by ILM peeling and the healing processes, and not damage to the RNFL itself. Hisatomi et al. [19] examined retinal changes after vitrectomy with ILM peeling by using a cynomolgus monkey model and focused on surgical damages of ILM peeling for a long observational period of 3 years. Their ultrastructural studies showed that most of the ILM peeling area was covered with glial cells during the wound healing processes and that retinal changes were found comparable with DONFL appearance, which was clinically observed with OCT. However, the mechanism underlying such changes observed 1 month or more after vitreous surgery has yet to be fully elucidated. Moreover, Müller cells span across the entire retina. Thus, if DONFL appearance is ILM peeling-associated damage to the Müller cells, then a localized lesion within the inner sensory retina is not explicable.
3. Involvement of Anoikis in the Pathogenesis of DONFL Appearance
There are two types of cells: anchorage-dependent and anchorage-independent cells. Anchorage-dependent cells proliferate while adhering to the culture vessel, and many cells, such as epithelial cells, belong to this category. On the other hand, cultured cells that proliferate while floating in the medium are known as anchorage-independent cells, such as blood cells. The shape of the whole body is maintained by adhesion between cells that constitute the whole body, and these cells are anchorage-dependent. Blood cells dispersed throughout the whole body are anchorage-independent. Anchorage-dependent cells undergo apoptosis when they are detached from proximal cells or the basement membrane and become suspended. This is probably a form of cellular suicide (i.e., programmed cell death) that naturally occurs to prevent the detached cells from migrating to other organs and disrupting the homeostasis of the surrounding tissues. Apoptosis that occurs when anchorage-dependent cells detach from the surrounding extracellular matrix (i.e., the loss of cell anchorage) is termed anoikis [20,21,22]. For many anchorage-dependent cells, adhesion to the extracellular matrix is an essential function/characteristic for survival and proliferation. However, cancer cells reportedly become anoikis resistant, and the resulting anchorage independence is thought to allow cancer cells to evade apoptosis and become metastatic [23,24]. Moreover, anchorage-dependent cells require attachment to solid substrates, such as basement membranes, and this cell adhesion is reportedly mediated by integrins [25]. Once integrin binding to basement membranes is disrupted, cells become suspended. To date, more than 25 types of integrins have been confirmed. When certain types of integrins are released from the substrates, signals that induce anoikis are transmitted to the nucleus through various kinases, such as focal adhesion kinase, thereby leading to anoikis [26]. The types of integrins that trigger anoikis differ according to cell types. Retinal ganglion cells express focal adhesion kinase [27,28], and the expression of integrins has reportedly been detected in the ILM and neural retina [25]. Therefore, ILM peeling may cause integrins to trigger anoikis by transmitting signals that induce anoikis to the nuclei of ganglion cells through focal adhesion kinase.
3.1. Anoikis and Eye Diseases
3.2. Anoikis and Glial Cells
3.3. β. A3/A1-Crystallin and Anoikis
3.4. E-Cadherin and Anoikis
3.5. Anoikis and Retinal Ganglion Cells
3.6. Neurogenesis in the Macular Region
Although the fovea centralis constantly collects light and is continually stressed by light, its morphology and function are maintained without cell depletion throughout the life cycle of the cell. The fovea centralis also has anatomical features of a tissue stem cell niche, i.e., avascular, hypoxic, and concave. Based on the hypothesis that undifferentiated cells, such as retinal stem cells, are present in the fovea centralis, and nerve regeneration may be occurring, we conducted an immunohistological study of monkey foveal retinas [77]. After preparing frozen tissue sections of the retina, including the foveal pit, immunostaining was performed for glial fibrillary acidic protein (GFAP), nestin, vimentin, neuron-specific class III β-tubulin (Tuj-1), arrestin 4, neurofilament, CD117, CD44, and Ki67, followed by fluorescence and/or confocal microscopy examinations. Immunostaining of the tissue sections enabled clear observation of strongly GFAP-positive cells that corresponded to the inner-half layer of the foveolar Müller cell cone. The surface layer of the foveal slope was partially contained with GFAP and vimentin. Tuj-1-positive cells were observed in the innermost layer of the foveolar retina, which spanned to the surrounding ganglion cell layer. Moreover, colocalization of Tuj-1 and GFAP was observed at the foveal pit. The coexpression of CD117 and CD44 was found in the interphotoreceptor matrix of the fovea. The foveolar cone stained positive for both nestin and arrestin 4. However, the photoreceptor layer outside of the foveola displayed weak staining for nestin. Colocalization of nestin and vimentin was observed in the inner half of the Henle layer, while colocalization of nestin and neurofilament was observed in the outer half, predominantly. Scattered Ki67-positive cells were observed in the cellular processes of the outer plexiform layer and the ganglion cell layer around the foveolar (Figure 3A,B). The Müller cell cone was divided into GFAP-strongly positive cells, presumably astrocytes, in the inner layer and nestin-positive/GFAP-weakly positive radial glia-like cells in the outer layer. These findings indicated that groups of such undifferentiated cells in the foveola might be involved in maintaining morphology and regeneration. Moreover, the precursor cells of TUJ-1-positive ganglion cells in the fovea started to divide, playing a role in homeostatic regeneration in the macular region. The pathogenesis of an idiopathic MH is tangential vitreomacular traction, which is generated by a detached perifoveal posterior vitreous membrane. There is some possibility that an MH might be related to anoikis of these undifferentiated cells.
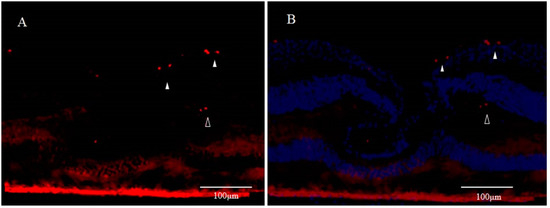
It is generally accepted that apoptosis occurs in a newly formed cell but is unlikely to occur in a senescent cell. In the adult hippocampus, most of the newly generated cells are eliminated by apoptosis, possibly because of their failure to integrate properly into neural networks [35]. Among the thousands of new neurons that integrate into the adult olfactory bulb each day, 50% are eliminated through apoptosis [78]. The continuous generation of new neurons in the adult brain is counterbalanced by accompanying cell death in the same regions [79]. As stated above, DONFL appearance is considered to be due to anoikis in a neuropoietic area around the fovea centralis; i.e., DONFL appearance may be due to anoikis that takes place when retinal stem cells present in the fovea centralis differentiate into ganglion cells. This might be the reason why retinal function was somewhat maintained after DONFL appearance occurred.
The findings in recent studies have demonstrated the presence of neural stem cells in the hippocampus and subventricular zone of the brain [80,81]. Neuroblasts migrate from the subventricular zone to the olfactory bulb along an astrocyte tube termed the ‘rostral migratory stream’ [82,83,84]. DONFL appearance is likely to be defects caused by anoikis in neuronal chains migrating along astrocytes in the Müller cell cone when neurons lose scaffolds for chain migration due to ILM peeling.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22041724
