Marine sponges (porifera) have proved to be a prolific source of unique bioactive secondary metabolites, among which the alkaloids occupy a special place in terms of unprecedented structures and outstanding biological activities. Identification of active cytotoxic alkaloids extracted from marine animals, particularly sponges, is an important strive, due to lack of knowledge on traditional experiential and ethnopharmacology investigations. In this report, a comprehensive survey of demospongian bioactive alkaloids in the range 1987–2020 had been performed with a special emphasis on the potent cytotoxic activity. Different resources and databases had been investigated, including Scifinder (database for the chemical literature) CAS (Chemical Abstract Service) search, web of science, Marin Lit (marine natural products research) database. More than 230 representatives of different classes of alkaloids had been reviewed and classified, different genera belonging to the phylum porifera had been shown to be a prolific source of alkaloidal molecules, including Agelas sp., Suberea sp., Mycale sp., Haliclona sp., Epipolasis sp., Monanchora sp., Crambe sp., Reniera sp., and Xestospongia sp., among others. The sufficient production of alkaloids derived from sponges is a prosperous approach that requires more attention in future studies to consider the constraints regarding the supply of drugs, attained from marine organisms.
- cytotoxicity
- alkaloids
- sponges
- marine drugs
- secondary metabolites
1. Introduction
Marine alkaloids present unique chemical structures that have been widely distributed among marine organisms. Some of them represent derivative molecules of the commonly encountered terrestrial alkaloids, whereas others show unprecedented novel structures confined to the marine systems. Their purification, structure elucidation, stereochemistry, chemical modification, synthesis, and pharmaceutical activity have acquired outstanding interdisciplinary attention from various fields of research aside from chemistry, including physiology, and pharmacology, ecology, biotechnology [1]. Alkaloids represent one of the most important classes of natural products that are widely distributed among different biological sources, including plants, animals, fungi, cyanobacteria, actinomycetes, dinoflagellates, red algae, cnidarians, and bryozoans; however, their presence in marine invertebrates as major constituents is limited to specific phylum, including some sponge genera, ascidians, mollusks, red algae, and bryozoans. Although the exact physiological function of alkaloids remains unclear, many of them had been developed as defense chemical weapons against predation—this is of great importance, especially for vulnerable sessile organisms, like sponges, and consequently, they are expected to be very potent molecules demonstrating toxicity at low doses [2,3,4].
This class of compounds demonstrates potent biological activities that can be considered as lead compounds for the development of potent antibiotics, antifungal, antiviral, anti-inflammatory, antimalarial, immune-modulating, or neuro-suppressive [5,6,7,8,9,10,11,12]. They also demonstrated promising cytotoxic activity versus diverse types of cancer cells [13,14]. Sponges (porifera), phylogenetically the oldest metazoan, have been recognized as a precious origin of unique secondary metabolites. About 5000 sponge species had been identified and classified with major groups belonging to Demospongiae [15]. They demonstrated wide distribution from intertidal coastal regions to great depths up to 8000 m depth [16]. The outstanding secondary metabolites produced by sponges are assumed to be the result of a combination of various factors, including the sessile nature of the organism, the porous nature of the body, and the environmental and ecological factors surrounding the organism [17]. As a consequence, it is obvious that these indefensible immobile organisms were provided by potent allelopathic factors that lead to the biosynthesis of many new drugs.
2. Alkaloid Classification
2.1. Acridine Alkaloids
Acridine nucleus (C13H9N) is a polycyclic heteroarene in which one of the central CH groups is replaced by a nitrogen atom. Chemical investigation of the Dercitus sp., collected from the Bahamas at 160 depth, led to the isolation of a violet pigment, identified as dercitin (1); cytotoxic evaluation of this compound against a panel of cell lines revealed potent cytotoxic activity in nanomolar concentration [23]. Investigation of different sponge samples belonging to genus Xestospongia collected from Indonesia, and New Guinea had led to the isolation of neoamphimedine (2), 5-methoxyneoamphimedine (3), amphimedine (4), neoamphimedine Z (5), alpkinidine (6). Compounds 2–6 were identified as bisannulated acridines. Based on results, compounds 2,3, and 6 showed selective activity for solid tumors—among them, 2 was the most potent, and 3 showed the most selectivity for solid tumors [24]. Figure 1 illustrates the chemical structures of compounds 1–6; Table 1 summarizes the cytotoxic evaluation of compounds 1–6.
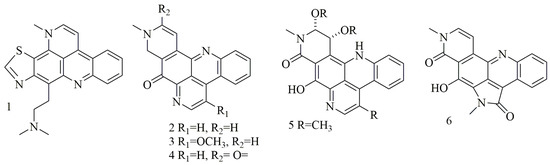
Figure 1. Structures of acridine alkaloids isolated from sponges.
Table 1. Cytotoxic activity of acridine alkaloids.
| Compound | Cell Line (IC50 values µM) |
Source | Place of Collection |
Ref. | |
|---|---|---|---|---|---|
| 1 | dercitin | P388 = 0.081 A-549 = 0.075 HT-29 = 0.063 HL-60 = 0.150 HL-60/AR = 0.240 |
Dercitus sp. | Bahamas | [23] |
| 2 | neoamphimedine | L1210 = 7.6 C38 = 7.6 H116 = 7.6 H125 = 7.6 CEM = 7.6 CFU-GM = 7.6 |
Xestospongia sp. | Indonesia and Papua | [24] |
| 3 | 5-methoxyneoamphimedine | L1210 = 72.8 C38 = 72.8 H116 = 72.8 H125 = 72.8 CEM = 72.8 CFU-GM = 72.8 |
|||
| 4 | amphimedine | L1210 = 11.9 C38 = 11.9 CFU-GM = 11.9 |
|||
| 5 | neoamphimedine Z | - | |||
| 6 | alpkinidine | L1210 = 362 C38 = 362 CFU-GM = 362 |
2.2. β-Carboline Alkaloid
Four β-carboline alkaloids 1,2,3,4-tetrahydronorharman-1-one (7) acanthomine A (8), annomontine (9), and ingenine E (10), were isolated from Acanthostrongylophora ingens. Compounds 7–10 showed the most potent cytotoxicity [25]. Figure 2 iIllustrates the chemical structures of compounds 7–10; Table 2 summarizes the cytotoxic evaluation of compounds 7–10.
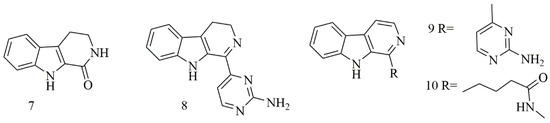
Figure 2. Structures of β-carboline alkaloids isolated from sponges.
Table 2. Cytotoxic activity of β-carboline alkaloids.
| Compound | Cell Line (IC50 µM) |
Source | Place of Collection |
Ref. | |
|---|---|---|---|---|---|
| 7 | 1,2,3,4-tetrahydronorharman-1-one | MCF7 = 44.4 HCT116 = 40.0 A549 = 54.3 |
Acanthostrongylophora ingens | Sulawesi Island in Indonesia | [25] |
| 8 | acanthomine A2 | MCF7 = 10.6 HCT116 = 2.2 A549 = 7.3 |
|||
| 9 | annomontine1 | MCF7 = 4.6 HCT116 = 1.5 A549 = 4.1 |
|||
| 10 | ingenine E | MCF7 = 13.1 HCT116 = 2.5 A549 = 8.0 |
2.3. Bromotyrosine Alkaloids
Qun Göthel et al. reported three bromotyrosine alkaloids identified as 14-debromo-11-deoxyfistularin-3 (11), aplysinin A (12), and aplysinin B (13) from the sponge Aplysina lacunosa collected from Stirrup Cay in the Bahamas. Compounds 11–12 revealed a unique, five-membered oxazole ring with a spiro atom linked with the bromocyclohexa-diene ring [26]. Investigation of different sponge samples belonging to genus Hexadella sp., Jaspis sp. and Bubaris sp., collected from Indonesia by Tarazona, and co-workers led to the isolation and identification of new bromotyrosine alkaloids 14–15. Aplyzanzine B (14) was isolated from Jaspis sp., and Bubaris sp., whereas Anomoian B (15) was isolated from Hexadella sp. have shown potent cytotoxic activity against three human tumor cell lines A549, HT-29, and MDA-MB-231 (6.1, 1.6, and 7.8 μM, respectively) [27].
In another study, ma’edamines C (16) and ma’edamines D (17) were isolated by Japanese researchers from the Okinawan marine sponge Suberea sp. compounds 16–17 revealed cyclization of the side-chain nitrogen of tyrosine to add a quaternary pyridinium nucleus to the structure. Both compounds have shown selective cytotoxicity against murine leukemia L1210 cell line though, and they did not express cytotoxicity against KB cell line [28]. Investigation of the marine sponge Psammoclemma sp. collected from Bommie Bay, Queensland, Australia, led to the isolation of psammaplysene C (18) and psammaplysene D (19). Compounds 18–19 showed C6C3N moiety in one of their bromotyrosine uints instead of the conventional C6C2N arrangement [29]; both compounds showed potent cytotoxic activities. Another bromotyrosyn alkaloids were isolated from the Fijian sponge Druinella sp. These 10 bromotyrosine alkaloids 20–29, were identified as purealidin S (20), purpuramine J (21). PurealidinQ (22), aplysamine 2 (23), purpureamine I (24), aerophobin2 (25), aerophobin1 (26), purealidin J (27), araplysillin1 (28), and araplysillin 2 (29). Compounds 25–27 revealed replacing the other bromobenzene moiety commonly encountered in bromotyrosine alkaloids with an imidazole ring. Compounds 20–29 were evaluated for their cytotoxicity against two cell lines showing potent to moderate activities. Among them, compound 21 was the most potent cytotoxic compound [30]. Suberedamine A (30) and suberedamine B (31) were isolated from Suberea sp. These two new cytotoxic bromotyrosine alkaloids exhibited potent cytotoxic activity against L1210 and KB cell lines [31]. Figure 3 illustrates the chemical structures of compounds 11–31.
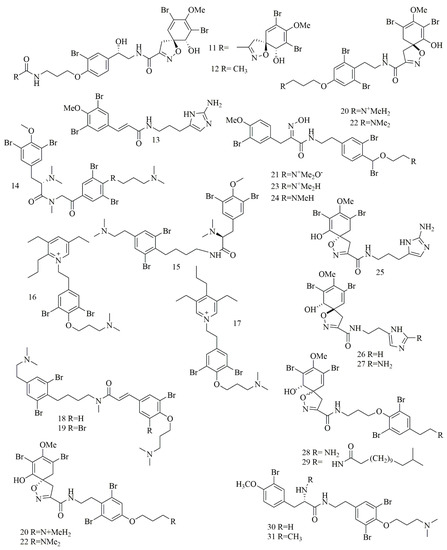
Figure 3. Structures of bromotyrosine alkaloids isolated from sponges.
2.4. Dibrominated and Brominated Alkaloids
Tilvi et al. reported three new pyrrole-2-aminoimidazole alkaloids from the Agelas dendromorpha collected from New Caledonia. Cytotoxic investigation of the isolated compounds revealed that only one compound identified as Agelastatin E (32) that showed 100% activity at 3 and 30 µM against KB cell lines [32]. In Shaala et al. a dibrominated alkaloid, aerothionin (33) with potent cytotoxicity against HeLa cells was identified from the sponge, Suberea sp., collected from the Red Sea in Yanbu, Saudi Arabia [33]. Two brominated indolosulfonic acid derivatives were reported from the hydroalcoholic extract of the Psammoclemma sp., collected from New Caledonia. The compounds were identified as echinosulfonic acid D (34) and echinosulfonic acid B (35) based on extensive LC/MS/MS analysis besides conventional 1 and 2 D NMR analysis. Both compounds showed potent cytotoxicity against KB cells with equal IC50 of 2 µg/mL [34]. Figure 4 illustrates the chemical structures of compounds 32–35.

Figure 4. Dibrominated and brominated alkaloids from marine sponges.
This entry is adapted from the peer-reviewed paper 10.3390/biom11020258
