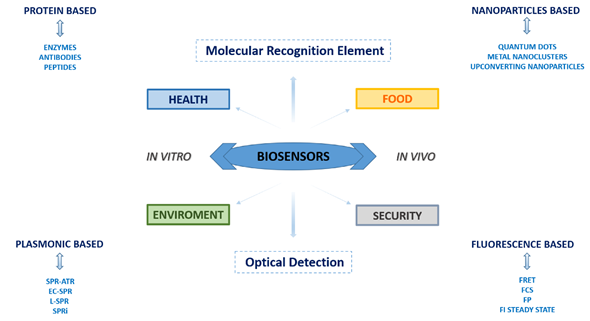
Over the years, biosensors have acquired increasing importance in a wide range of applications due to synergistic studies of various scientific disciplines, determining their great commercial potential and revealing how nanotechnology and biotechnology can be strictly connected. In the present scenario, biosensors have increased their detection limit and sensitivity unthinkable until a few years ago. The most widely used biosensors are optical-based devices such as surface plasmon resonance (SPR)-based biosensors and fluorescence-based biosensors.
- biosensor
- SPR
- fluorescence
- food
- security
- health
- environment
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Today the wide selection of available biosensors results to be segmented both on the basis of utilization and technology. Based on the used sensing technology, the extended array of biosensors can be classified into the following groups: (a) electrochemical biosensors; (b) optical biosensors; (c) piezoelectric biosensors; (d) thermal biosensors, and (e) nanomechanical biosensors.
Firstly, it is useful to restate that a biosensor can be considered as an analytical device incorporating a biological sensing element able to specifically bind to a substrate and turn this event into a measurable and quantifiable signal.
Usually, a biosensor device results composed of at least of three principal elements: (1) a “biological element” that recognizes the molecular target and, consequently, upon the binding of the target molecule, it generates a detectable signal; (2) a “transducer” that is able to highlight the generated signal; (3) an amplifier, that is able to quantify and transfer the signal to the operator (see Figure 1).
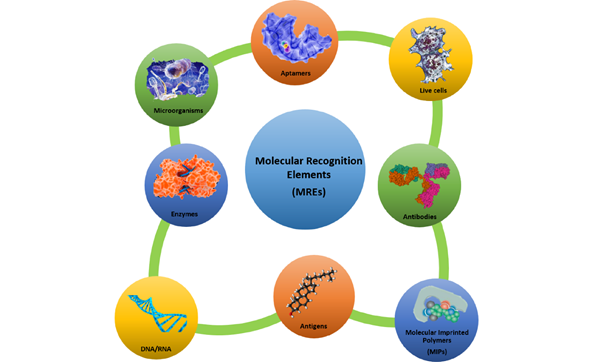
The use of an appropriate biological sensing element such as an enzyme, a protein, a nucleic acid sequence, an antibody, a microorganism, a part of a tissue, a cell, etc. is the most important step in the design of a biosensor. In fact, biological molecules possess special structural and functional features (such as high specificity and selectivity towards a target substrate), and they provide numerous advantages if used as molecular recognition elements (MREs) (see Figure 2). In addition, it is possible to overexpress them in vector systems to obtain large amounts of recombinant biomolecules, and it is also possible to genetically manipulate them for improving their structural and/or functional properties.
Figure 1. Schematic representation of the disclosed topics.
Figure 2. Molecular recognition elements—an overview. A selective overview of molecular recognition elements: protein receptors, enzymes, antibodies, nucleic acids, molecular imprinting polymer, cells, microorganisms, and aptamers.
In order to be successful whatever the nature and the quantity of the target analyte to be measured, a biosensor must possess at least some of the following features: (1) the biological sensing element must be highly specific for the target analyte; (2) it should be stable respect to some physical parameters such as pH and temperature variations; (3) it should be able to measure target analytes in complex real matrices with marginal pre-treatment steps of the sample; (4) the sensing response should be fast, accurate and reproducible (especially referred for early detection and diagnostics analyses); (5) it should be easily miniaturized and easy to use by semi-skilled operators [1].
Developing new biosensors that possess the above-described properties is of great relevance since they can be applied for tracing contamination and/or manipulation occurring in the food marketplace, such as foodborne pathogens, toxins of different origin, antioxidants, preservatives, and other potentially dangerous chemicals. Biosensors are a precious tool also for monitoring environmental pollutants and toxic molecules (ranging from pesticides and herbicides to aromatic compounds, and metal ions) dispersed in the atmosphere, water, and soil. Issues related to human security are also increasing in the last decade due to terrorist threats. Consequently, devices able to detect the presence of explosive substances are required in places like ports and airports, arenas, and institutional or government buildings. Finally, in the health field, biosensors are nowadays ubiquitous, being spread across biomedical research and clinical practice. The rapid and precise detection of many analytes, ranging from molecular disease-associated biomarkers to inflammation mediators, small metabolites, neurotransmitters, hormones, enzymes, etc., have crucial importance in terms of basic disease knowledge, as well as of drug design and diagnostics.
2. Surface Plasmon Resonance (SPR) Based Biosensors
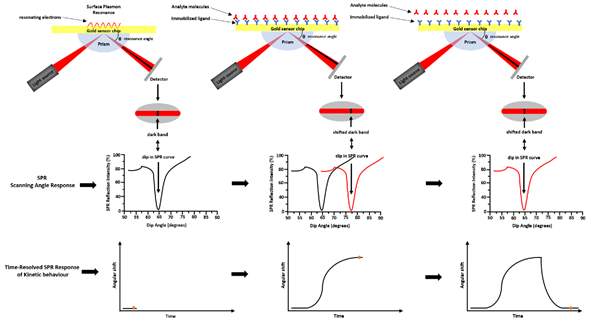
SPR is a technique based on the optoelectronic phenomenon that occurs when a visible or near-infrared light is incident upon a metal surface, such as Ag, Au, Cu, and Al. The radiation will through a specific prism and collimated to a detector (photodiode array) at the definite refractive index (RI) [2]. Changing the incidence angle changes the outcoming light until it reaches a critical angle. This phenomenon is called total internal reflection (TIR). When the frequency of the incident light is equal to the resonance frequency of the metal, it occurs an energy transfer from the photon of the light to the surface electrons of the metal. As consequence, the electrons move and generate an electrical wave (200 nm deep) called plasmon [3]. The surface plasmon resonance phenomenon takes place at a defined frequency of the light/angle of incidence, and it depends on the RI close to the metal surface that changes with the mass on the chip surface. The binding of molecules, within the range of the electric field, changes the mass on the chip surface and it perturbs the plasmon changing the resonance wavelength. The most widely used SPR detection method was based on the Kretschmann—Raether attenuated total reflection (ATR) configuration (see Figure 3). By the Kretschmann configuration, the dielectric constant changes of the medium near a metal film’s surface were detected by measuring the intensity changes of the reflected beam. Changing the geometry configurations, the light wavelength, and sensor surface, several SPR hybrid methods were designed, such as electrochemical surface-plasmon resonance (EC-SPR), localized surface plasmon resonance (LSPR), and SPR imaging (SPRi). In EC-SPR, a thin metal film is placed on the substrate to stimulate surface plasmons. It operates as an electrode for electrochemical detection, by providing information about the electrochemical and optical properties of the films [4–7]. Electrochemical configuration, in combination with SPR, can be used to study the kinetic reactions of biomolecules in the presence of electric fields.
Figure 3. Surface plasmon resonance—ATR via the Kretschmann configuration. Light is focused onto a metal film through a glass prism and the subsequent reflection is detected. At a certain incident angle (or resonance angle), the plasmons resonate at the same light frequency, resulting in the absorption of light at that angle. This determines a dark line in the reflected beam. That dark line contains a wealth of information. The resonance angle can be obtained by observing a dip in SPR reflection intensity. A shift in the reflectivity curve represents a molecular binding event taking place on or near the metal film, or a conformational change in the molecules bound to the film. By monitoring, this shift vs. time is possible to study the molecular binding events and binding kinetics.
Recently, EC-SPR has evolved in another hybrid technique, the SPR scanning electrochemical microscopy (SECM) [8,9]. SPR-SECM combines the sensitivity and resolution of SPR with the measure of the local electrochemical behavior of liquid/solid, liquid/gas, and liquid/liquid interfaces. The electrochemical signals are acquired using a precise ultramicroelectrode tip that scans the substrate region of interest. Changes in the recorded current depend on the distance between the electrode tip and substrate surface. This approach allows us to obtain the image of surfaces with information on topology and reactivity through moving the tip across surfaces. In LSPR is utilized a surface composed of nanomaterial with a dimension smaller than the wavelength of light.
The refractive index changes are induced by the size and the shape of the metal nanostructure and they can be used to monitor molecular binding events [10–12]. Controlling the size and shape of nanoparticles and the dielectric constant of the substrate, it is possible to modify and tune the LSPR characteristics [13–20]. Combining the dark-field (optical scattering) microscopy with the LSPR it is possible to evaluate local changes in the refractive index due to molecule binding events. The wavelength scanning (wavelength-shift measurement) approach is typically used to evaluate the absorption, scattered, or transmitted intensity from immobilized nanoparticles [21–23]. The nanoparticle size determines a highly confined electromagnetic field and defines the LSPR technique as sensitive to a single molecule. In fact, smaller nanoparticles represent an advantage for the detection of single molecules in bio-sensing approaches.
Gold and silver nanoparticles (NPs) exhibit LSPR at visible as well as near-infrared frequencies, with sharp peaks in their spectral absorbance. The absorption wavelength of the LSP is characteristic of the type of material and it is strongly dependent on the dielectric environment, the size, and the shape of the NPs [24]. One major disadvantage, however, is that LSPR sensors are prone to interference because they respond not only to refractive index variations but also to non-specific binding events. These interactions can severely compromise the measurements when working in complex matrices, and hence they limit the applicability and impact of their utilization [25]. Surface plasmon resonance microscopy (SPRM), also called surface SPRi, is a label-free method that combines the surface plasmon resonance of metallic surfaces with imaging of the metallic surface. It is an advanced version of classical SPR analysis, where the sample is monitored without a label through the use of a CCD camera. The heterogeneity of the refractive index of the metallic surface imparts high contrast images, caused by the shift in the resonance angle. SPRM can achieve a sub-nanometers thickness sensitivity and lateral resolution achieves values of micrometers scale. SPRM measurements can be made in real-time, such as measuring binding kinetics of membrane proteins in single cells, or DNA hybridization. The main advantage of SPRi technology with the use of a CCD camera is the simultaneously recording of sensorgrams and SPR images for the analysis of hundreds of interactions. To increase the throughput of standard SPR biosensors, Rothenhausler and Knoll [26–29] developed the SPR microscopy or imaging method. This approach suffered from a reduced sensitivity compared to conventional SPR. The SPRi's additional value is to offer the opportunity to visualize a whole biochip via a CCD camera. The biochips are prepared in an array format where each spot simultaneously provides a quantity of biological information. The CCD camera provides images in real-time from hundreds of spots. The acquired images show local changes on the chip surface and provide detailed information on molecular interactions and kinetic processes.
Another approach called high-resolution SPR imaging combines the CCD camera resolution with an inverted optical microscope, equipped with a high numerical aperture oil immersion objective [30–32]. This configuration permits a pixel-by-pixel tracking of the reflectivity in the SPR images. Each of these pixels accordingly produces an SPR curve and the image is framed using the SPR minimum angle information.
All mentioned technologies are widely applied in biosensor applications, and in the next paragraph, we will describe the applications of the emergent SPR biosensor in food, environment, security, and health (Table 1).
Table 1. List of analyte targets detected by surface plasmon resonance (SPR)-based sensors.
|
Analyte |
Method |
Substrate/Sensing Layer |
LoD |
Ref. |
|
Food |
||||
|
Campylobacter jejuni |
SPR-ATR |
Au-coated thin glass/Antibody |
1.0 × 103 CFU/ml |
[33] |
|
“ ” |
SPR-ATR |
Au-coated thin glass/Antibody |
1.1 × 105 CFU/ml |
[38] |
|
Salmonella typhimurium |
SPR-ATR |
C18 Au-coated thin glass/Antibody |
2.5 × 105 CFU/ml |
[34] |
|
“ ” |
SPR-ATR |
Au-coated thin glass/Antibody |
1.0 × 105 CFU/ml |
[35] |
|
Salmonella enteritidis |
SPR-ATR |
C18 Au-coated thin glass/Antibody |
2.5 × 108 CFU/ml |
[34] |
|
“ ” |
SPR-ATR |
Au-coated thin glass/Antibody |
1.0 × 106 CFU/ml |
[37] |
|
Salmonella choleraesuis |
SPR-ATR |
Au-coated thin glass/Antibody |
4.4 × 104 CFU/ml |
[38] |
|
Escherichia coli O157:H7 |
SPR-ATR |
Au-coated thin glass/Antibody |
1.0 × 105 CFU/ml |
[35] |
|
“ ” |
SPR-ATR |
Au-coated thin glass/Antibody |
1.4 × 104 CFU/ml |
[38] |
|
Yersinia enterocolitica |
SPR-ATR |
Au-coated thin glass/Antibody |
1.0 × 105 CFU/ml |
[35] |
|
Legionella pneumophila |
SPR-ATR |
Au-coated thin glass/Antibody |
1.0 × 105 CFU/ml |
[35] |
|
Listeria monocytogenes |
SPR-ATR |
Au-coated thin glass/Antibody |
1.0 × 107 CFU/ml |
[36] |
|
“ ” |
SPR-ATR |
Au-coated thin glass/Antibody |
1.0 × 106 CFU/ml |
[37] |
|
“ ” |
SPR-ATR |
Au-coated thin glass/Antibody |
3.5 × 103 CFU/ml |
[38] |
|
Aflatoxin B1 |
SPR-ATR |
Au-coated thin glass/Antigen |
0.2 ng/gr |
[39] |
|
Fumonisin B1 |
SPR-ATR |
Au-coated thin glass/Antibody |
50 ng/mL |
[40] |
|
Enterotoxin B |
SPR-ATR |
Au-coated thin glass/Antibody |
100 fM |
[41] |
|
Ricin |
SPR-ATR |
Au-coated thin glass/Antibody |
200 ng/mL |
[42] |
|
Abrin |
SPR-ATR |
Au-coated thin glass/Antibody |
75 ng/mL |
[43] |
|
Tetrodotoxin |
SPR-ATR |
Au-coated thin glass/Antigen |
0.3 ng/mL |
[44] |
|
Tylosin |
SPR-ATR |
Au-coated thin glass/Antigen |
2.5 µg/Kg |
[45] |
|
Phenol |
SPR-ATR |
Au-coated thin glass/Antigen |
5 ppm |
[46] |
|
Ascorbic acid |
SPR-ATR |
Fiber optic core/Ag PANI MIP |
1.28 × 10−10 M |
[47] |
|
Environment |
||||
|
Chlorpyrifos |
EC-SPR |
Au-coated thin glass/MIP Fe3O4-PDA NPs |
0.76 nM |
[48] |
|
Atrazine |
SPR-ATR |
Fiber optic core/Ag MIP |
1.92 × 10−14 |
[50] |
|
VOCs (1-octanol) |
SPRi |
Au-coated thin glass/Biomimetic peptides |
375 ppb |
[51] |
|
Pb2+ |
SPR-ATR |
Au-coated thin glass/Ag-CS |
30 ppb |
[52] |
|
“ ” |
SPR-ATR |
Au-coated thin glass/Ag-CS-GO |
30 ppb |
[53] |
|
Co2+ |
SPR-ATR |
Au-coated thin glass/PAR-Cs-GO |
10 ppb |
[54] |
|
Cu2+ |
SPR-ATR |
Au-coated thin glass/CTA-NCC-GO |
0.01 ppm |
[55] |
|
Security |
||||
|
TNT |
SPR-ATR |
Au-coated thin glass/Antigen |
0.002 ng/mL |
[57] |
|
“ ” |
SPR-ATR |
Au-coated thin glass/PAMAM-antigen |
110 pg/mL |
[58] |
|
“ ” |
SPR-ATR |
Fiber optic core/Au MIP |
5.1 × 10−5 M |
[59] |
|
Capsaicinoids |
SPR-ATR |
Au-coated thin glass/OEG-antigen |
150 ppb |
[60] |
|
Homovanillic acid |
SPR-ATR |
Au-coated thin glass/Apten antigen |
150 ppb |
[61] |
|
Health |
||||
|
Newcastle disease virus |
LSPR |
Fiber optic Ex-TFGs /Au-NP-Antibody |
25 pg/mL |
[62] |
|
HIV-1 virus |
LSPR |
Au NP-coated thin glass/Antibody |
200 fg/mL |
[63] |
|
Dengue virus |
SPR-ATR |
Au-coated thin glass/Antibody |
2 × 104 particles/mL |
[64] |
|
Avian influenza A H7N9 virus |
SPR-ATR |
Au-coated thin glass/Antibody |
402 copies/mL |
[65] |
|
Progesterone |
SPR-ATR |
Au-coated thin glass/Antigen |
170 pg/mL |
[66] |
|
Estradiol |
SPR-ATR |
Au-coated thin glass/Antigen |
3.5 ng/mL |
[67] |
|
Cortisol |
SPR-ATR |
Au-coated thin glass/OEG-antigen |
49 pg/mL |
[68] |
|
Testosterone |
SPR-ATR |
Au-coated thin glass/OEG-antigen |
15.4 pg/mL |
[69] |
|
Sulfamethazine |
SPR-ATR |
Au-coated thin glass/Antigen |
0.015 µg/mL−1 |
[70] |
|
Sulfadiazine |
SPR-ATR |
Au-coated thin glass/Antigen |
0.052 µg/mL−1 |
[70] |
|
Clenbuterol |
SPR-ATR |
Au-coated thin glass/Antigen |
0.4 ng/mL−1 |
[71] |
|
Ethinylestradiol |
SPR-ATR |
Au-coated thin glass/Antigen |
0.5 ng/mL−1 |
[71] |
|
Enrofloxacin |
SPR-ATR |
Au-coated thin glass/Antigen |
1.2 ng/mL−1 |
[71] |
|
Tetracycline |
SPR-ATR |
Fiber optic core/Ag MIP |
0.01 µM |
[72] |
|
“ ” |
SPR-ATR/LSPR |
Fiber optic core/Ag NP/MIP |
2.2 × 10−9 M |
[73] |
|
Oxytetracycline |
SPR-ATR |
Fiber optic core/Ag MIP |
0.01 µM |
[72] |
|
Erythromycin |
SPR-ATR |
Fiber optic core/Ag MIP |
6.2 × 10−8 M |
[74] |
|
Vitamin B3 |
SPR-ATR |
Fiber optic core/Ag MIP |
0.5 mg/mL |
[75] |
|
Nicotine |
SPR-ATR |
Fiber optic core PMMA/Au MIP |
1.86 pM |
[78] |
|
Epstain-Barr Virus |
SPR-ATR |
Au-coated thin glass/Antigen |
0.1 ng/mL |
[81] |
|
Interleukin-6 |
SPR-ATR |
Fiber optic core/Antibody |
0.92 ng/mL |
[82] |
|
Prostate-specific antigen |
SPR-ATR |
Au-coated thin glass/Antibody |
8.5 pM |
[84] |
|
Carbohydrate antigen 15-3 |
SPR-ATR |
Au-coated thin glass/Antibody |
0.025 U/mL |
[85] |
|
Carcinoembryonic antigen |
EC-SPR |
Au-coated thin glass/Antibody |
0.5 ng/mL−1 |
[86] |
|
C-reactive protein |
SPR-ATR |
Au-coated thin glass/Antigen |
0.1 ng/µl |
[87] |
|
HER2 |
SPR-ATR |
Au-coated thin glass/Antibody |
11 ng/mL |
[88] |
|
Progesterone receptor |
SPR-ATR |
Au-coated thin glass/Antigen |
3.56 ng/mL |
[90] |
|
Metalloproteinases-9 |
SPR-ATR |
Au-coated thin glass/Antibody |
8 pg/mL |
[91] |
|
BRCA1 |
SPRi |
Au-coated thin glass/Au NPs/DNA |
1 pM |
[92] |
|
Cholesterol |
SPR-FTIR |
Au-coated thin glass/Cells |
9 mg/gr |
[99] |
|
Volatile compound (octanal) |
SPRi |
Au-coated thin glass/Cells |
0.1 mM |
[100] |
2.1. Food
One of the most common applications of SPR biosensors is in food safety, where biosensors have been developed for several classes of contaminants as foodborne pathogens, mycotoxins, plant and bio-marine toxins, toxic chemicals (mostly of anthropogenic source), preservatives, and anti-oxidants (Table 1).
For pathogen detection, different SPR biosensors have been developed. For example, Wei et al. [33] used the SPREETATM SPR system (Texas Instruments) to detect Campylobacter jejuni using polyclonal antibody immobilized directly on the sensor surface. The assay showed a sensitivity of 1 × 103 CFU/mL.
Barlen et al. [34] used an SPR device to detect Salmonella typhimurium (2.5 × 105 CFU/mL) and S. enteritidis (2.5 × 108 CFU/mL). Oh et al. [35] developed an SPR-based protein chip with immobilized monoclonal antibodies against S. typhimurium, E. coli O157:H7, Yersinia enterocolitica, and Legionella pneumophila. Hearty et al. [36] produced a murine monoclonal antibody against the surface-located L. monocytogenes internalin A (InA). The obtained LoD was of 1 × 107 CFU/mL.
Koubová et al. [37] designed a home-made device that was able to detect 1 × 106 CFU/mL of L. monocytogenes and S. enteritidis. Taylor et al. [38] created an eight-channel SPR sensor that allowed the simultaneal detection of E. coli O157:H7 (1.4 × 104 CFU/mL), L. monocytogenes (3.5 × 103 CFU/mL), C. ejuni (1.1 × 105 CFU/mL), and S. choleraesuis (4.4 × 104 CFU/mL).
SPR was successfully used for the detection of small molecules such as bacterial and dinoflagellate toxins, mycotoxins, and plant toxins. An indirect test to detect aflatoxin B1 is also reported [39]. The produced SPR immunosensor allowed us to detect the presence of fumonisin B1 in milk samples with an LoD of 50 ng/mL [40].
Naimushin et al. [41] designed an SPR platform to detect the presence of sub-nanomolar concentrations of enterotoxin B, produced by Staphylococcus aureus in milk, seawater, and mushrooms.
Ricin represents one of the most potent plant toxins. Its detection was performed by many methods, but not with SPR until recently. Feltis et al. [42] developed a homemade biosensor to detect ricin at low concentrations with respect to the minimum lethal dose (200 ng/mL).
Abrin, is a highly potent and lethal type II ribosome-inactivating toxin from Abrus precatorius. Its structure is similar to the structure of ricin and it has the same biochemical mechanism of action. It was developed a very sensitive assay (75 ng/mL) through the production of two human monoclonal antibodies, able to bind this toxin with high affinity and specificity [43]. Taylor et al. [44] reported the quantitative antibody-based detection of tetrodotoxin (TTX) through an inhibition assay using an SPR sensor. The assay was based on the use of an anti-TTX antibody sensing surface and it allowed a detection limit of 0.3 ng/mL.
In recent years, there has been an increase in the use of tylosin in apiculture due to resistance to oxytetracycline. Caldow et al. [45] reported an SPR assay to detect the presence of tylosin with a detection limit of 2.5μg/kg. In addition, an interesting approach for the detection of phenol employing living cells was presented by Choi et al. [46]. They fabricated a sensor surface containing the E. coli O157:H7 strain. The cellular damage associated with the phenol presence induced a change of SPR signal. The detection limit of phenol was 5 ppm [46]. Ascorbic acid is a commonly available nutrient that has anti-oxidizing properties. It is largely used in industrial food processing as a preservative. Excess of ascorbic acid in food produces gastric problems. A polyaniline molecular imprinting polymers (PANI) MIP-based fiber optic sensor exploiting the principle of SPR was reported (LoD of 1.28 × 10−10 M) [47].
2.2. Environment
The SPR technique is largely applied for the detection of pesticides, herbicides, aromatic compounds, chemical mixtures, and toxic metal ions that are responsible for environmental contaminations (Table 1).
One of the most widespread classes of pollutants is acetylcholinesterase inhibitors pesticides (organophosphate and carbamate) widely used for pest and insect control in agriculture, livestock, and domestic uses.
Several SPR optical biosensors have been developed to detect acetylcholinesterase (AChE) inhibitors. However, the small size of the inhibitors produces a low shift in resonance and the consequentially a poor sensitivity. To overcome this problem in the SPR assays are applied nanoparticles (NPs) that promote a significant shift in the angle of plasmon resonance.
Chlorpyrifos (CPF) is one of the most diffuse pesticides, and Yao et al. [48] reported an innovative detection method based on the synthesis of magnetic MIP-NPs. MIPs present recognition sites for CPF. NPs were synthesized using Fe3O4. The Fe3O4-NPs showed a high molecular weight and magnetic features. Integrating the CPF-imprinted Fe3O4 NPs to an SPR chip resulted in a significant signal amplification due to the high molecular weight of NPs. The SPR biosensor showed a detection limit for CPF of 0.76 nM.
Atrazine, a member of the triazine class, is an herbicide. It is used for the control of the broadleaf weeds in crops [49]. Due to its toxic nature, it can affect the ecosystem and human health causing cancer or reproductive abnormalities. The monitoring of this molecule in the environment (air and water samples) is of fundamental interest. Agrawal et al. developed a method for the detection of atrazine by coupling the molecular imprinting technology (MIT) with the SPR approach over the use of an optical fiber substrate [50]. The MIPS, able to recognize the atrazine, were immobilized onto a fiber optic substrate. The developed SPR sensor showed to be very sensitive (LoD of 1.92 × 10−14 M).
Monitoring the presence of harmful chemicals such as benzene, toluene, ethylbenzene, xylene, and volatile organic compounds (VOCs), is an interesting and emerging field of SPR applications. In fact, SPRi may provide comprehensive information on the composition of VOCs besides a simple detection. The integration of SPRi to the micro-gas chromatography system allows for simultaneous separation and multidimensional detection of target chemicals in a gas mixture. Brenet et al. [51] have developed an SPRi chip for VOCs sensing in the gas phase. The developed sensor showed high selectivity and the capability to discriminate between different VOCs differing only for a single carbon atom.
Metal ions contaminations represent still a serious problem for the environment and health. Exposure to metal ions can cause harm and affect human health. The coupling of graphene oxide (GO) nanoparticles with the SPR method improved the detection capabilities of toxic metal ions. Lokman et al. [52] developed an SPR sensor for the detection of Pb2+ with high sensitivity of 0.77 ppm−1. They enhanced the sensitivity of the SPR sensor by developing a gold-chitosan-graphene oxide (Au/CS/GO) nanostructured thin film [53]. To detect Co2+ Saleviter et al. [54] prepared an active layer immobilized 4-(2-pyridylazo) resorcinol in a chitosan–graphene oxide composite (PAR-Cs-GO). The obtained sensor was able to detect Co2+ as low as 10 ppb. Daniyal et al. [55] developed an SPR sensor to detect Cu2+ with an LoD of 0.01 ppm. They prepared a sensor surface altering the nanocrystalline cellulose by hexadecyltrimethylammonium bromide and GO composite (CTA-NCC/GO).
2.3. Security
The SPR methodology is deeply applied also in the field of human security. In fact, SPR based devices able to sense and detect explosives such as the trinitrotoluene (TNT) and/or chemical warfare agents (CWAs) such as the lachrymators (like the capsaicin, have been developed in the last decade (Table 1).
The most diffused SPR approach is the immunosensor because the antibodies have the capability of detecting low molecular weight compounds like 2,4,6-TNT and capsaicin.
The detection of TNT by an SPR immunosensor was reported by Zeck et al. [56], using an indirect competitive assay. The surface sensor was immobilized a 2,4,6-trinitrophenyl-keyhole limpet hemocyanin conjugate. As detection molecules, commercially available monoclonal antibodies against 2,4,6-TNT were utilized [57]. Another approach to detect TNT used a competitive immunoassay based on a dendrimer-modified SPR surface [58]. A thiol SAM combined with a poly(amidoamine) (PAMAM) dendrimer provided the support structure for attachment of dinitrotoluene-keyhole limpet hemocyanin conjugate (immobilized antigen). Using a monoclonal antibody as a detection molecule, an LoD of 110 pg/mL was achieved [58].
Combining fiber optic SPR and MIP technique, Cennamo et al. [59] developed a highly sensitive TNT SPR sensor. The fiber optic surface was realized by the coating of 60 nm thick gold film over the core of the fiber, the MIP was immobilized on the gold surface. The developed sensor showed an LoD of 5.1 × 10−5 M. Using an SPR immunosensor approach Onodera et al. [60] detected the capsaicinoids. To recognize a vanillyl group of capsaicinoids a polyclonal antibody against homovanilic acid (CCH) was developed. An indirect competitive assay was performed by immobilizing the capsaicin analogs via a SAM on the surface of the sensor. Different capsaicinoids, homovanillic acid, and vanillylamine (4-hydroxy-3-methoxybenzylamine) were used for the sensor chip on which vanillylamine was immobilized. The developed indirect competitive assay shows an LoD of 150 ppb [61].
2.4. Health
The application of new methods of analysis in the health field is characterized by the typology of the analytes and by the heterogeneity of the sample (matrix) to analyze. SPR is an exciting tool for health diagnosis and clinical treatment monitoring. In fact, SPR biosensors were developed for the detection of small molecules like drugs (steroid hormones, cocaine, ecstasy, heroin, amphetamine, antibiotic, sulfamethazine, vitamin, nicotine, melamine, erythromycin, and dopamine), polypeptides, proteins (growth factors, cancer biomarkers, antibodies, and serum proteins), DNA molecules and whole organisms (bacteria and virus) (Table 1). In particular, SPR-immune biosensors have been largely applied to identify biomolecules of interest, taking advantage of the large availability of specific antibodies from the marketplace and the simplicity to produce ad hoc antibodies.
The capability of SPR to analyze several types of biological fluids and tissue matrices (saliva, blood, whole cell, and etc.) and the possibility to monitor in real-time the association-dissociation process of biological molecules, have prompt the development of SPR applications for in vivo assays. The application of SPR for in vivo analysis has permitted us to clarify several molecular aspects of cellular functioning.
The detection of pathogenic viral agents takes advantage of the highly sensitive and selective SPR methods. In particular, analytical methods based on PSPR or LSPR are widely utilized. The detection of the Newcastle disease virus (NDV) was possible by an LSPR immunosensor developed by Luo et al. [62]. They coated excessively tilted fiber grating (Ex-TFG) with AuNP, and monitoring the resonance wavelength shift, achieved an LoD of ~25 pg/mL.
The HIV virus-like particle detection was achieved by an immunosensor based on the LSPR mechanism [63]. The diagnosis of dengue viral infection was possible by a rapid propagating surface plasmon resonance (PSPR)-based immunoassay, where a neutravidin-biotin monoclonal antibody (the sensing element) was immobilized on a thin gold film. An LoD of 2 × 104 particles/mL was obtained [64]. Avian influenza A H7N9 was detected by Chang et al. [65]. They developed an intensity-modulated surface plasmon resonance (IM-SPR)-based immunosensor. The observed LoD, in samples spiked, was 402 copies/mL.
For the family of steroid hormones, estradiol and progesterone were detected by SPR with an online in-tube solid-phase microextraction (SPME) system, to monitor estrogenic cycles in cows, with an LoD of 3.5 ng/mL [66] for the progesterone and an LoD of 170 pg/mL for the estradiol [67].
The detections of cortisol and testosterone in human saliva are of great interest because they are associated with hormonal disorders such as Addison’s disease and Cushing’s syndrome. Coupling the covalent immobilization of antibodies and OEG linker technology was possible to construct highly sensitive SPR immunoassays for both cortisol [68] and testosterone [69]. The LoD values obtained were 49 pg/mL and 15.4 pg/mL for cortisol and testosterone respectively.
The development of a high-throughput detection assay allowed the analysis of the bile that is a complex fluid because contains many different analytes of interest. Sulfamethazine and sulfadiazine were detected [70]. The same technology was, also applied for measurements of clenbuterol and ethinylestradiol in urine and sulfamethazine, sulfadiazine, and enrofloxacin in milk [71].
Another class of analytes is represented by antibiotics used for the prevention and treatment of several bacterial infections. Large amounts of antibiotics, however, may harm the human body via allergic symptoms and other diseases. Tetracycline (TC) and oxytetracycline (OTC) are two very diffuse antibiotics. A detection method coupling MIT and fiber optic SPR technique was developed coating an Ag thin film over the core of the optical fiber followed by a MIP TC/OTC layer. The sensor operation was checked for the tetracycline concentration range 0–0.96 µM and for the OTC concentration range 0–0.96 µM [72].
Shrivastav et al. [73] improved the sensitivity (an LoD of 2.2 × 10−9 M) of the TC sensor by incorporating the combined phenomenon of SPR and LSPR. The sensor was fabricated by including the Ag nanoparticle layer between Ag and MIP-TC layer [74].
Erythromycin (ERY) is another diffuse antibiotic used to reduce the activities of Gram-positive and Gram-negative bacteria. Its wide use results in its presence in foodstuffs and derivatives. The detection of ERY in an aqueous medium was allowed by an SPR sensor developed using ERY-MIP nanoparticles. The sensor was able to sense an ERY concentration range from 0.0 to 50 µM [74].
The SPR approach is also used for vitamin detection. The most important vitamin is vitamin B3, also known as niacin/3-pyridinecarboxamide, essential for maintaining healthy skin, proper breathing, and metabolism and to keep the nervous system fully functional. A molecular imprinted hydrogel-based SPR fiber optic sensor utilizing colloidal crystal templating was reported to detect the vitamin B3 (analyte concentration of 0 to 10 mg/mL) [75]. A similar approach was developed for riboflavin/vitamin B2, with a concentration range of 0–320 µg/mL [76].
Another interesting class of analytes is represented by drugs like cocaine, nicotine, ecstasy, heroin, and amphetamine. For drug detection, the most diffuse SPR method is the LSPR that uses a combination of antibodies and antigen-protein conjugates immobilized on the array [77].
Nicotine is reported to affect the nervous system which can result in paralysis and respiratory block. The detection of nicotine in human body fluid was performed by Cennamo et al. [78] using a fiber optic L-nicotine sensor. They coupled SPR and MIP on tapered PMMA plastic fiber. The sensor showed a response time of 10 min and an LoD of 1.86 pM [78].
Another application of SPR technology is the diagnostic screening of serum samples, epitope mapping, and protein expression profiling. Nagel et al. [79] restricted their SPR studies for serological detection of Lyme borrelioses to two widely used antigens. The whole proteins as well as two peptides, representing immunodominant domains, were used as capture probes. De Boer et al. [80] used an SPR platform that combines the microarray principle with SPR detection in one flow chamber. The microarray contained 144 different glycans derived from the human parasite Shistosoma mansoni and was used for the simultaneous detection of glycan-specific serum antibodies.
An SPR biosensor was used to detect antibodies directly from human blood serum against the immunoreactive peptide epitope of Epstain-Barr Virus (EBV) nuclear antigen. The detection limit was estimated to be 0.1 ng/mL, which is lower by an order of magnitude than the detection limit of Enzyme-Linked Immunosorbent Assay (ELISA) [81].
The serum components present in low concentration, like IgE or cytokines, may not be detected, but Battaglia et al. [82] demonstrated the detection of biologically relevant levels of the cytokine IL6 in cell culture media using an SPR sensor. To reduce the non-specific protein adsorption, the sensor surface was modified by a layer of NHS ester and 16-mercaptohexadecanoic. Weinhart et al. [83] suggested SAMs of linear polyglycerol derivates for gold surfaces.
The ability to detect biomarkers in blood samples is really important for clinical applications. However, biomarkers in blood samples are present in small concentrations. The SPR method, with the aid of nanoparticles, represents an interesting tool to overcome this issue. NPs-SPR sensors were developed for detection of prostate-specific antigen (tPSA) [84], carbohydrate antigen 15-3 (CA15-3) [85], carcinoembryonic antigen (CEA) [86], C-reactive protein (CRP) [87], human epidermal growth factor receptor 2 (HER2) [88], estrogenic receptor (ER) [89], progesterone receptor (PR) [90]. By using 40 nm nanoparticles conjugated with the PSA antibody, a tPSA assay was performed on 75% human serum at a detection limit of 0.29 ng/mL−1 (8.5 pM). C-reactive protein (CRP) is a principal blood serum biomarker for conventional inflammation, Jung et al. [87] developed a spectral SPR system to detect C-reactive protein (CRP) in human sera immobilizing the CRP monoclonal antibody to dextran functionalized gold surface.
An important biomarker for malignant tumor progression and metastasis is the human matrix metalloproteinases-9 (MMP-9). An SPR-based immunosensors for real-time and label-free detection of recombinant MMP-9 was reported by Mohseni et al. [91]. Combining the surface hybridization, surface ligation, and nanoparticle amplification for single-nucleotide polymorphism (SNP) genotyping in BRCA1 gene Li et al. [92] developed an SPR method to evaluate the presence of a single mismatch on BRCA1 gene by using nanoparticles with oligonucleotides complementary to the ligation probe DNA. They were able to detect the SNP at concentrations as low as 1 pM. The nanoparticles substantially helped to overcome the limitation of conventional SPR biosensors [93].
An additional interesting area of health is the in vivo monitoring of physiological phenomena such as cellular response, cell adhesion, and cellular products, as well as detection of cancer cells and bacterial cells. Since cells respond to stimulation of reactive molecules, the cell-molecule interaction cause changes in the SPR signal, and, of consequence, SPRi represents a suitable technology to reveal cell-molecules interactions [94–96].
Yanase et al. [96], developed an SPR sensor to detect the presence of intracellular events observed the changes in the size of the cell adhesion area. It has been observed that the value of RI near the plasma membrane, which could be determined by the accumulation and rearrangement of the proteins activated by the transduction of the intracellular signal, changes profoundly following exogenous stimuli.
The precise mechanism for cells to determine such large variations of RI is not yet fully understood. However, detections and/or analyses of cellular functions were studied by measuring the value of RI with respect to real-time adhesion and morphological changes in cells in response to various agents [97]. For example, the use of an infrared SPR sensor based on FTIR-SPR with Fourier transform has made it possible to know the changes in the biochemical composition of the membrane, such as cholesterol [98,99]. An SPR sensor with cells that express the olfactory receptor has been designed for the detection of volatile compounds [98–100], to detect the reactions of cancer cells against an anticancer drug [101,102] or small morphological changes that occur following the induction of apoptosis in cells [103].
This entry is adapted from the peer-reviewed paper 10.3390/s21030906