Plant NBR1 is a structural homolog and functional hybrid of mammalian autophagy receptors NBR1 and p62.
- autophagy
- selective autophagy receptors
- plants stress responses
- NBR1
1. Introduction
Autophagy is a highly conserved pathway in eukaryotes that recycles multiple cytoplasmic components under both normal and stress conditions such as starvation [1]. Induction of autophagy is initiated by the formation of an isolation membrane called phagophore that can extend to capture and sequester cytoplasmic components within a double-membrane vesicle termed autophagosome [2,3]. Mature autophagosomes can then fuse with the lysosomes or vacuoles for degradation of their cargo by resident hydrolases. The core machinery of autophagosome formation requires more than 40 largely conserved autophagy-related proteins (ATG). In vertebrates, these core autophagy components function in several physiologically continuous, but mechanistically distinct, steps and are organized into several functional complexes including: (i) the ULK (Unc-51 Like Autophagy Activating Kinase) complex with ULK1 and -2, ATG13, ATG101 and FIP200 (FAK Family Kinase-Interacting Protein of 200 kDa), (ii) the class III phosphoinositide 3-kinase (PI3K) complex I, with VPS34 (Vacuolar Protein Sorting 34), VPS15, Beclin1 and ATG14 for the nucleation and assembly of the initial phagophore membrane, (iii) the phosphatidylinositol-3-phosphate (PI3P)-binding ATG2A or -B and WIPI1–4 (WD Repeat Domain Phosphoinositide-Interacting Protein 1–4) complex, and iv) the two interrelated ubiquitin-like conjugation systems, ATG12–ATG5–ATG16 and ATG8–PE (phosphatidylethanolamine), which are required for the membrane elongation and expansion of the forming autophagosomes [4]. In addition, the ATG4 cysteine proteases process the precursors of ATG8 proteins for their lipidation and delipidation [4].
During cellular response to nutrient deprivation, autophagy usually involves non-selective uptake of cytoplasm into phagophores for bulk degradation of intracellular contents [5]. However, the broad roles of autophagy are primarily mediated by selective clearance of certain components [5]. In human cells, extensive studies have reported the selective autophagic degradation of aggregation-prone misfolded proteins and protein aggregates implicated in the pathology of various neurodegenerative diseases [5]. Furthermore, autophagy selectively degrades diverse organelles such as mitochondria, peroxisomes, lysosomes, endoplasmic reticulum (ER) and the nucleus, under various conditions [5]. Ubiquitin-like ATG8 plays a critical role in selective autophagy [6]. After attachment of the lipid PE to its carboxyl terminus through a conjugation pathway, ATG8 is both anchored in the membrane of autophagosomes and acts as a docking platform for the selective recruitment of cargos through a three-way interaction of selective autophagy receptors with both ATG8 and cargos [6]. Most selective autophagy receptors interact with membrane-anchored ATG8 through ATG8-interacting motifs (AIMs), which have the W/Y/F-X-X-L/I/V consensus core sequence [6]. AIMs of selective autophagy receptors bind a hydrophobic patch on ATG8 known as the AIM docking site [7]. A new class of selective autophagy receptors have been recently identified that interact with ATG8 through ubiquitin-interacting motif (UIM)-like sequences for high-affinity binding to an ATG8 interaction site different from the AIM docking site [8,9].
Autophagy has been extensively analyzed over the past two decades or so in Arabidopsis and, to a lesser extent, in other plants. Using genetic and molecular approaches, these extensive studies have established an important role of autophagy in almost all aspects of plant life, particularly in plant stress responses [10,11]. Autophagosome biogenesis and ATG gene expression are both induced under diverse abiotic stress conditions including nutrient starvation, heat, salt, drought and oxidative stresses [12,13,14,15,16,17]. Autophagy mutants and transgenic silencing lines display increased sensitivity to nutrient starvation and abiotic stresses when compared to wild-type plants [12,13,14,15,16,17]. In addition, plant mutants or transgenic silencing lines for autophagy are altered in response to virulent and avirulent biotrophic pathogens including pathogen-induced hypersensitive cell death [18,19,20,21,22,23]. Autophagy-deficient mutants are hypersusceptible to necrotrophic pathogens [19,24]. Furthermore, autophagy affects plant interaction with viral pathogens through regulation of antiviral RNA silencing, targeting degradation of viral proteins and other processes [20,25,26,27,28]. Autophagy also plays important roles in plant growth and development including root growth, leaf senescence, pollen and endosperm development [22,29,30,31,32].
Over the past ten years or so, a substantial number of selective autophagy receptors have been identified, characterized and functionally analyzed in plants [33,34] (Table 1; Figure 1). While a few of these autophagy receptors in plants are evolutionarily conserved with homologs in other types of organisms, most of them are plant-specific or even plant species-specific. The cargos recognized by these plant selective autophagy receptors include not only misfolded, nonactive and otherwise unwanted cellular components, but also regulatory and signaling factors [33,34]. Characterization of these plant autophagy receptors and their cargos have provided important new insights into the critical roles of autophagy in plant responses to a broad spectrum of biotic and abiotic stresses. Several recent reviews have covered selective autophagy in plants that also include discussion on well-studied selective autophagy receptors from plants [34,35,36].
.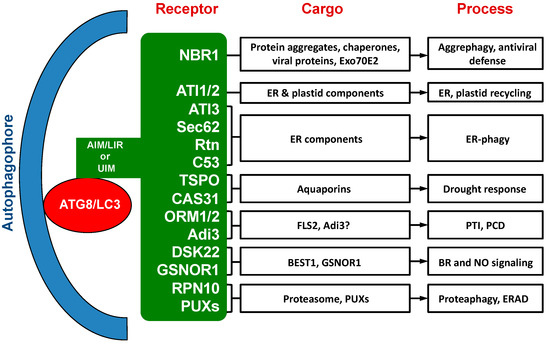 Figure 1. Cargo proteins and involved processes of selective autophagy receptors in plants.
Figure 1. Cargo proteins and involved processes of selective autophagy receptors in plants.
Table 1. Interacting ATG8 (autophagy-related protein 8) isoforms and recognition motifs of selective autophagy from plants.
| Receptor | Interacting ATG8 Isoform | Recognition Motif |
|---|---|---|
| NBR1 | ATG8s | AIM |
| ATI1/2 | ATG8f, ATG8h | AIM |
| ATI3s | ATG8a, ATG8f | AIM |
| Sec62 | ATG8e | AIM |
| ZmRtn1/2 | ZmATG8a | AIM |
| C53 | ATG8a–g, ATG8i | AIM |
| TSPO | ATG8e | AIM |
| MtCAS31 | MtATG8a | AIM |
| ORM1/2 | ATG8a, ATG8d, ATG8e, ATG8i | AIM |
| SlAdi3 | SlATG8h | AIM |
| DSK2 | ATG8e | AIM |
| GSNOR1 | ATG8s | AIM |
| RPN10 | ATGa, ATG8e, ATG8f, ATG8i | UIM |
| PUXs | ATG8a, ATG8e | UIM |
2. NBR1 (Neighbor of BRCA1)
Among the identified autophagy receptors in plants, NBR1 has been most extensively characterized. Plant NBR1 is a structural homolog and functional hybrid of mammalian autophagy receptors NBR1 and p62 [51,52]. Both mammalian p62 and NBR1 proteins contain an N-terminal PB1 (Phox and Bem1p) domain, a ZZ-type zinc finger domain, an LC3-interacting region or LIR motif (also known as AIM motif in yeast and plants) and a C-terminal UBA (ubiquitin-associated) domain [53]. In addition, there is a highly conserved globular domain characterized by the presence of four highly conserved tryptophan residues in NBR1 but not in p62 [53]. Only metazoans contain both p62 and NBR1 homologs, while other eukaryotic organisms only have NBR homologs [53]. Plant NBR1 homologs lack the coiled coil domain of mammalian NBR1 but have two C-terminal UBA domains [37]. Model plant Arabidopsis contains a single gene encoding an NBR1 homolog, which, however, can homo-oligomerize through the N-terminal PB1 domain like p62 [37]. Only the C-terminal UBA domain of the two UBA domains of Arabidopsis NBR1 binds ubiquitin [37].
The biological functions of plant NBR1 have been analyzed through characterization of nbr1 mutants or transgenic silencing lines. Arabidopsis nbr1 knockout mutants are normal in growth and development under normal growth conditions. The nbr1 mutants are also normal in general autophagy and in the selective clearance of peroxisomes, mitochondria, or the endoplasmic reticulum (ER) [16,54,55,56]. Plant NBR1 is not essential either for age- and darkness-induced senescence but may modulate growth or senescence under certain conditions such as short-day growth condition or under mineral deficiency [16,52,57]. The Arabidopsis nbr1 mutants also respond normally to a necrotrophic pathogen [16]. However, loss of Arabidopsis NBR1 gene function compromise plant tolerance to heat, oxidative, salt, and drought stresses [16,55]. The role of NBR1 in plant abiotic stress tolerance is mediated by selective autophagy based on its dependent on the interaction with ATG8 and is associated with the clearance of aggregation-prone misfolded proteins and protein aggregates [16] (Figure 1). In Arabidopsis, NBR1 also plays a role in resistance to the bacterial pathogen Pseudomonas syringae by suppressing the establishment of an aqueous extracellular space (“water-soaking”) [58]. More recent studies have further revealed important roles of plant NBR1 in the modulation of plant heat stress memory, plant–viral interaction and other stress-associated processes. These new roles of NBR1-mediated selective autophagy will be discussed in more detail here.Very often, plants can be subjected repeatedly to a stress condition such as high temperature and need to balance between growth recovery and keeping stress memory for better survival when faced with a subsequent harsher stress [59]. Autophagy is induced in plants by moderate heat stress and targets a large number of proteins including specific heat shock proteins (HSPs) for degradation during the recovery phase after the end of heat stress, leading to reduced heat stress memory [59]. These target proteins include HSP90.1 and its interacting partner ROF1/AFKBP62 (rotamase FKBP 1), a plant homolog of mammalian FKBP4/FKBP52 [16,60]. The HSP90.1-ROF1 complex remains in the cytoplasm under normal conditions but binds heat shock transcription factor HSFA2 and translocates to the nucleus to activate heat-responsive gene expression following exposure to heat stress [61]. Degradation of HSP90.1 and ROF1 by NBR1-mediated selective autophagy attenuates HSFA2-dependent induction of HSP genes and represses the response to heat stress [60]. Indeed, the nbr1 loss-of-function mutants is stronger in heat stress memory [60]. These results indicate that plant NBR1 plays complex roles in plant heat stress responses. It promotes basal heat tolerance mostly through autophagic degradation of misfolded/denatured proteins or protein aggregates to mitigate heat-induced proteotoxicity (Figure 2). After the end of heat stress, NBR1-mediated selective autophagy targets degradation of specific HSPs to reduce heat stress memory, probably to promote growth recovery but also downregulate acquired heat tolerance to a potential subsequent heat stress (Figure 2).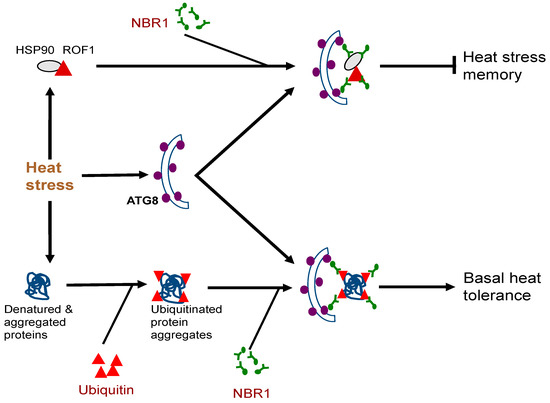 Figure 2. Complex roles of plant NBR1 (Neighbor of BRCA1) in plant heat stress responses. It mediates autophagic degradation of misfolded/denatured proteins or protein aggregates to mitigate heat-induced proteotoxicity to promote basal heat tolerance. NBR1-mediated selective autophagy also targets degradation of specific heat shock proteins (HSPs) such as HSP90 to reduce heat stress memory.
Figure 2. Complex roles of plant NBR1 (Neighbor of BRCA1) in plant heat stress responses. It mediates autophagic degradation of misfolded/denatured proteins or protein aggregates to mitigate heat-induced proteotoxicity to promote basal heat tolerance. NBR1-mediated selective autophagy also targets degradation of specific heat shock proteins (HSPs) such as HSP90 to reduce heat stress memory.
Autophagy plays an important role in plant–virus interactions. Previous studies have demonstrated that autophagy regulates virus-induced hypersensitive cell death and targets degradation of plant and viral proteins associated with dsRNA-induced RNA silencing [20,25]. More recent studies have revealed that NBR1-mediated selective autophagy targets degradation of specific viral proteins to suppress viral infection. In the study with Cauliflower mosaic virus (CaMV), it has been shown that NBR1-mediated selective autophagy targets nonassembled and virus particle-forming capsid proteins for degradation to restrict the establishment of CaMV infection [26]. To counter the antiviral defense mechanism, the CaMV-induced virus factory inclusions sequester the viral proteins and coordinate particle assembly and storage to protect capsid proteins against autophagic destruction [26]. NBR1 also targets the viral RNA silencing suppressor helper-component proteinase (HCpro), presumably in association with virus-induced RNA granules, to suppress accumulation of Turnip mosaic virus (TuMV), a positive-stranded RNA potyvirus [27]. Again, as counter defense mechanisms, several viral proteins have evolved the activity to antagonize NBR1-dependent autophagy. These results demonstrate the critical role of NBR1-mediated selective autophagy in plant antiviral defense and the potential viral strategies to evade and adapt autophagic processes for successful infection.Recent studies have also demonstrated other cargos recognized and potentially targeted by NBR1 and further illustrate a broad role of the autophagy receptor in plant metabolism and stress responses. For example, Arabidopsis NBR1 is a selective receptor for Exo70E2 during autophagy in Arabidopsis [57]. Exo70E2 is a subunit of the exocyst complex, which directs the secretory vesicles of exocytosis from the Golgi complex to specific locations on the plasma membrane and to mediate their tethering and localization to the membrane immediately before fusion [62]. In Arabidopsis, there is a double-membrane organelle termed the exocyst-positive organelle (EXPO), which may be involved in mediating unconventional protein secretion in plants [63,64]. Exo70E2 is a marker for EXPO [63]. Upon induction of autophagy, Exo70E2-GFP positive EXPOs and autophagosome were colocalized and delivered to vacuoles for degradation in transgenic Arabidopsis plants [63]. Arabidopsis NBR1 specifically interacted and recruited Exo70E2 or its EXPO to ATG8-positive autophagosomes in a manner independent of its UBA domains [57]. Knockout of the NBR1 gene significantly reduced the vacuolar delivery of Exo70E2 or EXPO upon autophagic induction [57], supporting that the Arabidopsis NBR1-mediated selective autophagy pathway is involved in the vacuolar delivery of Exo70E2 or EXPO in plant autophagy.Arabidopsis NBR1 also interacts with members of the plant-specific LSU (response to Low SUlfur) protein family, which are induced by sulfur (S) deficiency, suggesting a possible role of NBR1 in plant S nutrient responses [65]. Indeed, S deficiency induces autophagy and the transcription of NBR1. NBR1 overexpression alters plant gene expression in response to the low S conditions [66]. Furthermore, Arabidopsis seedlings overexpressing NBR1 have significantly shorter roots than wild type when grown under S deficient conditions in the presence of TOR kinase inhibitors [66]. Arabidopsis NBR1 also interacts with three regulatory proteins of the abscisic acid (ABA) pathway (ABI3, ABI4 and ABI5) in planta [67]. NBR1 interaction with ABI5, but not ABI3 or ABI4, requires its UBA domain [67]. It is likely that ABI5, but not ABI3 or ABI4, requires ubiquitination prior to interaction with NBR1. It would be of interest to determine whether NBR1 binding of ABI3, ABI4 and ABI5 causes their autophagic degradation and affects their protein levels and ABA signaling in plants.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22031013
