The brain-gut-microbiota axis is a bidirectional system enabling gut microorgan-isms to communicate with the central nervous system (CNS), and the CNS with the gut . The mechanisms of signal transmission are complex and not fully understood, but include neural, endocrine, immune and metabolic pathways.
- gut microbiota
- gut microbiome
- gut-brain axis
- functional bowel disorders
- irritable bowel syn-drome
- antibiotics
- probiotics
1. Introduction
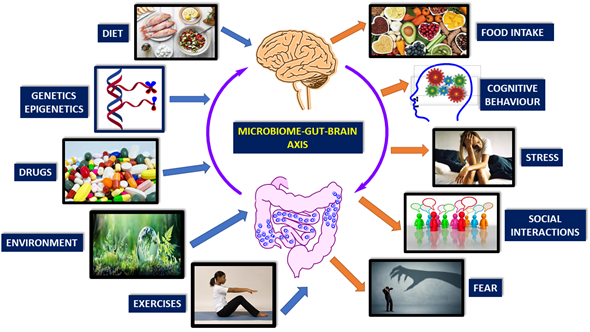
There are many factors affecting microbiome-gut-brain-axis. These are diet, genetics, drugs, environment, exercise, cognitive behavior, stress, social interactions, and fear (Figure 1) [1][2][3][4][5][6][7][8][9].
Gut microbes are capable of producing most neurotransmitters found in the human brain. While these neurotransmitters primarily act locally in the gut, modulating the enteric nervous system, there is also undeniable evidence indicating that gut microbes can influence CNS through multiple mechanisms. The treatment with probiotic Bifidobacteria for instance, can increase the amount of tryptophan, the precursor of serotonin [10]. Some Lactobacilli species alter gamma-aminobutyric acid (GABA) metabolism and change brain GABA receptor expression and behavior [11]. Preclinical studies show that the vagus nerve is the main route for exerting the effects of gut microbiota on CNS. Lactobacillus rhamnosus has a central effect in animals and this was ameliorated by vagotomy [12]. In fact, patients with a history of vagotomy have diminished risk for certain neurological diseases [13]. Synthesis and release of neurotransmitters from bacteria have been reported: Lactobacillus and Bifidobacterium species can produce GABA; Escherichia, Bacillus and Saccharomyces spp. can produce noradrenaline; Candida, Streptococcus, Escherichia and Enterococcus spp. can produce serotonin; Bacillus can produce dopamine; Lactobacillus can produce acetylcholine [14][15][16]. Although these neurotransmitters can cross inflamed intestinal mucosal barrier, they cannot cross blood–brain-barrier (BBB) in healthy conditions. Another way of gut-brain interaction is the stimulation of hypothalamic-pituitary-adrenal (HPA) axis, which induces cortisol secretion. This system is the main stressor system in the body and it is mainly regulated by gut-HPA axis [17][18][19][20][21][22][23][24][25][26][27][28]. Psychological or physical stress can affect HPA axis and subsequently gut microbiota/barrier function (e.g., IBS) [29].
Post-infectious IBS is a prototype for gut-brain axis disorders. Water-born gastroenteritis outbreak occurred in United States and people affected from this E.coli infection later developed IBS-like symptoms including co-morbid depression, anxiety disorder [30].
Figure 1. Brain-gut-microbiome axis is a dynamic, interactive network. Factors affecting the communication of these elements are mainly genetics, diet, and lifestyle (Modified from reference [31]).
2. Pathways of Communication
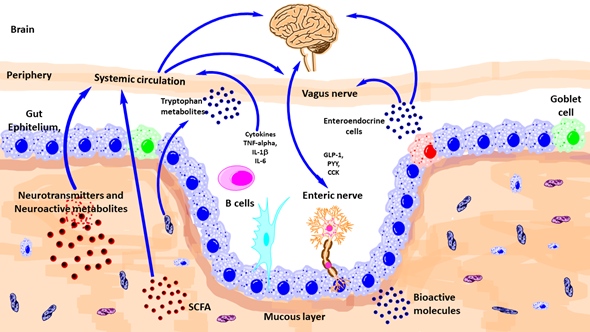
There are diverse ways of communication between gut microbiota and brain such as autonomic nervous system, vagus nerve, enteric nervous system, neurotransmitters, and immune system (Figure 2).
Figure 2. Schematic outlining the various known pathways of communication between the gut-microbiota and the brain. CCK, Cholecystokinin; GLp-1, glucagon-like peptide-1; IL, interleukin; PYY, peptide YY; TNF, tumor necrosis factor; SCFA, short-chain fatty acid. (Modified from reference [31]).
2.1. Autonomic Nervous System (ANS)
The autonomic nervous system (ANS) comprises the sympathetic and parasympathetic branches. Combined with activity from the enteric nervous system (ENS) and influenced by the CNS, the ANS is responsible for physiological homeostasis, as well as responding to endocrine, motor, autonomic, and behavioral areas. Gut microbiota communicate with ANS bidirectional both antagonistic and synergistically [32][33]. Afferent nerves carry information from visceral organs to CNS and from CNS, important survival messages sent towards peripheral organs. ANS acts as the most immediate responder in health and disease states [34][35][36][37][38][39][40][41]. Local GI autonomic activation can be stimulated by afferent feedback loops from the microbiota and CNS efferent modulation [42]. Microbiota-related metabolites such as tryptophan (and end products, e.g., serotonin-5HT), GABA, catecholamine’s mediate ANS related effects. Sympathetic innervation has post-ganglionic vasoconstrictor effects and also suppressive effects on gut secretions and motility. Intestinal mucus layer is regulated by sympathetic innervation, by modulating mucosal immune system, microbial composition, and function [43].
2.2. Vagus Nerve
It is the tenth cranial nerve and the longest in the body with extensive connections and networks with peripheral organs. The vagus exerts anti-inflammatory actions via medullary dorsal motor nucleus. The vagal modulation of macrophage action an important factor for the inflammation in inflammatory bowel disease (IBD) [44].
2.3. Enteric Nervous System (ENS)
At the interface of microbiota and host, there is a network of gut neurons called ENS. Anatomically divided as submucosal and myenteric plexus, ENS regulates gut motility and secretions [45]. Factors affecting neurodevelopment and health status of CNS may also affect ENS integrity. Gut microbiota influences development and function of ENS via pattern recognition receptors (PRR) and including Toll-like receptors (TLRs), especially TLR-2 and TLR-4. These TLRs are involved in the recognition of microbial molecules [46]. Bacteroides fragilis and the capsular exopolysaccharide, are good examples, which can influence ENS function [47]. L. rhamnosus strain (JB-1) performs this action via a G protein-coupled receptor-mediated pathway [31]. Recent data showed that stress-induced alterations in ENS activity, via stimulated acetylcholine release, were influenced by both maternal separation and the microbiota [48]. This proves that the microbiota may affect ENS-related gut dysfunction associated with early-life stress. ENS abnormalities are associated with life-threatening GI disorders including Hirsch sprung disease and chronic intestinal pseudo-obstruction [49]. Moreover, the ENS is also involved in disorders of the CNS, including ASD, Alzheimer’s disease (AD), and Parkinson’s disease (PD) [50].
2.4. Immune System
Gastrointestinal tract has the highest number of immune cells in the body and there is a delicate, complex communication with the gut microbiota [51]. Mucus produced by epithelial Goblet cells provide a barrier against contact with host cells and microbial elements. The gut microbiota influences regulation of subsets of immune cells including T helper (Th), T regulatory (Treg), natural killer (NK), mononuclear phagocytes, and innate lymphoid cells [52]. The mechanisms by which the gut microbiota influences innate and adaptive responses during health and disease is still being investigated.
Environmental factors affect immune function. The release of cytokines promotes the activation and recruitment of eosinophils, B cells, and mast cells. In addition to the traditional T-helper 2 pathway, secretion of IL-23 from antigen-presenting cells, such as dendritic cells, B cells, and macrophages, promotes T helper-cell 17 differentiation. Degranulation of these cells disrupt intestinal barrier and enteric nerves. This results in visceral hypersensitivity and dysmotility [53]. α4β7 gut homing T cells are a marker of intestinal inflammation in both functional dyspepsia and irritable bowel syndrome. The site and extend of intestinal immune activation can define the phenotype such as functional heartburn, functional dyspepsia, irritable bowel syndrome, functional constipation, or functional diarrhea [53] (Figure 3).
Figure 3. Mucosal immune system activation and functional gastrointestinal disorders. [53]. CRF, corticotrophin-releasing factor; ACTH, adrenocorticotropic hormone; Ig, immunoglobulin; IL, interleukin; TH, T-helper cell.
This entry is adapted from the peer-reviewed paper 10.3390/nu13020389
References
- Bear, T.L.K.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The Role of the Gut Microbiota in Dietary Interventions for Depression and Anxiety. Adv. Nutr. 2020, 11, 890–907, doi:10.1093/advances/nmaa016.
- Xu, F.; Fu, Y.; Sun, T.Y.; Jiang, Z.; Miao, Z.; Shuai, M.; Gou, W.; Ling, C.; Yang, J.; Wang, J.; et al. The interplay between host genetics and the gut microbiome reveals common and distinct microbiome features for complex human diseases. Microbiome 2020, 8, 145, doi:10.1186/s40168-020-00923-9.
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519.
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215, doi:10.1038/nature25973.
- Clark, A.; Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43, doi:10.1186/s12970-016-0155-6.
- Gareau, M.G. Microbiota-gut-brain axis and cognitive function. Adv. Exp. Med. Biol. 2014, 817, 357–371, doi:10.1007/978-1-4939-0897-4_16.
- Rea, K.; Dinan, T.G.; Cryan, J.F. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol. Stress 2016, 4, 23–33, doi:10.1016/j.ynstr.2016.03.001.
- Moeller, A.H.; Foerster, S.; Wilson, M.L.; Pusey, A.E.; Hahn, B.H.; Ochman, H. Social behavior shapes the chimpanzee pan-microbiome. Sci. Adv. 2016, 2, doi:10.1126/sciadv.1500997.
- Bharwani, A.; Mian, M.F.; Foster, J.A.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinol 2016, 63, 217–227, doi:10.1016/j.psyneuen.2015.10.001.
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteriainfantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174.
- Patterson, E.; Ryan, P.M.; Wiley, N.; Carafa, I.; Sherwin, E.; Moloney, G.; Franciosi, E.; Mandal, R.; Wishart, D.S.; Tuohy, K.; et al. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci. Rep. 2019, 9, 16323.
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Nat. Acad. Sci. USA 2011, 108, 16050–16055.
- Svensson, E.; Horvath-Puho, E.; Thomsen, R.W.; Djurhuus, J.C.; Pedersen, L.; Borghammer, P.; Sørensen, H.T. Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol. 2015, 78, 522–529.
- Cryan, J.F.; Dinan, T.G. Mind-alteringmicroorganisms: Theimpact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712, doi:10.1038/nrn3346.
- Galland, L. The gut microbiome and thebrain. J. Med. Food. 2014, 17, 1261–1272, doi:10.1089/jmf.2014.7000.
- Lyte, M. Microbial endocrinology in the microbiome-gut-brain axis: How bacterial production and utilization of neurochemi-cals influence behavior. PLoS Pathog. 2013, 9, e1003726, doi:10.1371/journal.ppat.1003726.
- Lyte, M. Microbial endocrinology and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014, 817, 3–24, doi:10.1007/978-1-4939-0897-4_1.
- Juruena, M.F.; Eror, F.; Cleare, A.J.; Young, A.H. The Role of Early Life Stress in HPA Axis and Anxiety. Adv. Exp. Med. Biol. 2020, 1191, 141–153, doi:10.1007/978-981-32-9705-0_9.
- vanBodegom, M.; Homberg, J.R.; Henckens, M.J.A.G. Modulation of the Hypothalamic-Pituitary-Adrenal Axis by Early Life Stress Exposure. Front. Cell Neurosci. 2017, 11, 87, doi:10.3389/fncel.2017.00087.
- García-León, M.Á.; Pérez-Mármol, J.M.; Gonzalez-Pérez, R.; García-Ríos, M.D.C.; Peralta-Ramírez, M.I. Relationship between resilience and stress: Perceived stress, stressful life events, HPA axis response during a stressful task and hair cortisol. Physiol. Behav.2019, 202, 87–93, doi:10.1016/j.physbeh.2019.02.001.
- Oyola, M.G.; Handa, R.J. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: Sex differences in regu-lation of stress responsivity. Stress 2017, 5, 476–494, doi:10.1080/10253890.2017.1369523.
- Shapero, B.G.; Curley, E.E.; Black, C.L.; Alloy, L.B. The interactive association of proximal life stress and cumulative HPA axis functioning with depressive symptoms. Depress. Anxiety 2019, 36, 1089-1101, doi:10.1002/da.22957.
- Roos, L.G.; Janson, J.; Sturmbauer, S.C.; Bennett, J.M.; Rohleder, N. Higher trait reappraisal predicts stronger HPA axis ha-bituation to repeated stress. Psychoneuroendocrinol 2019, 101, 12–18, doi:10.1016/j.psyneuen.2018.10.018.
- Young, E.S.; Doom, J.R.; Farrell, A.K.; Carlson, E.A.; Englund, M.M.; Miller, G.E.; Gunnar, M.R.; Roisman, G.I.; Simpson, J.A. Life stress and cortisol reactivity: An exploratory analysis of the effects of stress exposure across life on HPA-axis functioning. Dev. Psychopathol. 2020, 1, 12, doi:10.1017/S0954579419001779.
- Lovelock, D.F.; Deak, T. Acute stress imposed during adolescence has minimal effects on hypothalamic-pituitary-adrenal (HPA) axis sensitivity in adulthood in female Sprague Dawley rats. Physiol. Behav. 2020, 213, 112707, doi:10.1016/j.physbeh.2019.112707.
- Leistner, C.; Menke, A. Hypothalamic-pituitary-adrenal axis and stress. Handb. Clin. Neurol. 2020, 175, 55–64, doi:10.1016/B978-0-444-64123-6.00004-7.
- Starr, L.R.; Stroud, C.B.; Shaw, Z.A.; Vrshek-Schallhorn, S. Stress sensitization to depression following childhood adversity: Moderation by HPA axis and serotonergic multilocus profile scores. Dev. Psychopathol. 2020, 1–15, doi:10.1017/S0954579420000474.
- Bao, A.M.; Swaab, D.F. The human hypothalamus in mood disorders: The HPA axis in the center. IBRO Rep. 2018, 6, 45–53, doi:10.1016/j.ibror.2018.11.008.
- Cryan, J.F.; Dinan, T.G. Mind-alteringmicroorganisms: Theimpact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712.
- Lee, Y.Y.; Annamalai, C.; Rao, S.S.C. Post-Infectious Irritable Bowel Syndrome. Curr. Gastroenterol. Rep. 2017, 19, 56.
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013.
- Jänig, W. Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis;Cambridge University Press: Cam-bridge, UK, 2006.
- Mulak, A.; Bonaz, B. Irritable bowel syndrome: A model of the brain-gut interactions. Med. Sci. Monit. 2004, 10, RA55–RA62.
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938, doi:10.1172/JCI76304.
- Bienenstock, J.; Kunze, W.; Forsythe, P. Microbiota and the gut-brainaxis. Nutr. Rev. 2015, 73, 28–31, 2015, doi:10.1093/nutrit/nuv019.
- Bonaz, B.L.; Bernstein, C.N. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 2013, 144, 36–49, doi:10.1053/j.gastro.2012.10.003.
- Mayer, E.A.; Tillisch, K. The brain-gut axis in abdominal pain syndromes. Ann. Rev. Med. 2011, 62, 381–396, doi:10.1146/annurev-med-012309-103958.
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409, doi:10.1038/nrn2647.
- Chey, W.Y.; Jin, H.O.; Lee, M.H.; Sun, S.W.; Lee, K.Y. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am. J. Gastroenterol. 2001, 96, 1499–1506, doi:10.1111/j.1572-0241.2001.03804.x.
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463, doi:10.1016/j.cell.2013.11.024.
- O'Mahony, S.M.; Hyland, N.P.; Dinan, T.G.; Cryan, J.F. Maternal separation as a model of brain-gut axis dysfunction. Psy-chopharmacology 2011, 214, 71–88, doi:10.1007/s00213-010-2010-9.
- 59. O'Mahony, S.M.; Marchesi, J.R.; Scully,P.; Codling, C.; Ceolho, A.-M.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267, doi:10.1016/j.biopsych.2008.06.026.
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F.I.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403.
- Bonaz, B.L.; Bernstein, C.N. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 2013, 144, 36–49.
- Hyland, N.P.; Cryan, J.F. Microbe-host interactions: Influence of the gut microbiota on the enteric nervous system. Dev. Biol. 2016, 417, 182–187.
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352.
- Mao, Y.K.; Kasper, D.L.; Wang, B.; Forsythe, P.; Bienenstock, J.; Kunze, W.A. Bacteroidesfragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons. Nat. Commun. 2013, 4, 1465.
- De Palma, G.; Blennerhassett, P.; Lu, J.; Deng, Y.; Park, A.J.; Green, W.; Denou, E.; Silva, M.A.; Santacruz, A.; Sanz, Y.; et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat. Commun. 2015, 6, 7735.
- Gariepy, C. Intestinal Motility Disorders and Development of the Enteric Nervous System. Pediatr. Res. 2001, 49, 605–613.
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503.
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14.
- Ganal-Vonarburg, S.C.; Duerr, C.U. The interaction of intestinal microbiota and innate lymphoid cells in health and disease throughout life. Immunology 2020, 159, 39–51.
- Black, C.J.; Drossman, D.A.; Talley, N.J.; Ruddy, J.; Ford, A.C. Functional gastrointestinal disorders: Advances in understanding and management. Lancet 2020, 9, S0140–S6736.