Coumarins are a family of benzopyrones (1,2-benzopyrones or 2H-[1]benzopyran-2-ones), which represent an important family of oxygen-containing heterocycles, widely distributed in nature. Since coumarins have versatile applications, the synthesis trials of different structures of the coumarin-based scaffold were attempted. Among all the heterocycles built on α-pyrone moiety of coumarin, the furan ring was the only available structure in nature. Thus, it has inspired a lot of researchers to replace the oxygen with other heteroatoms. Wide varieties of heterocycles were constructed by a synthetic pathway to introduced furans, pyrroles, thiophenes, and selenophenes as a fused ring that characterized by a single heteroatom to the α-pyrone moiety of coumarin.
- coumarins
- benzopyrones
- five-membered aromatic heterocycles
1. Introduction
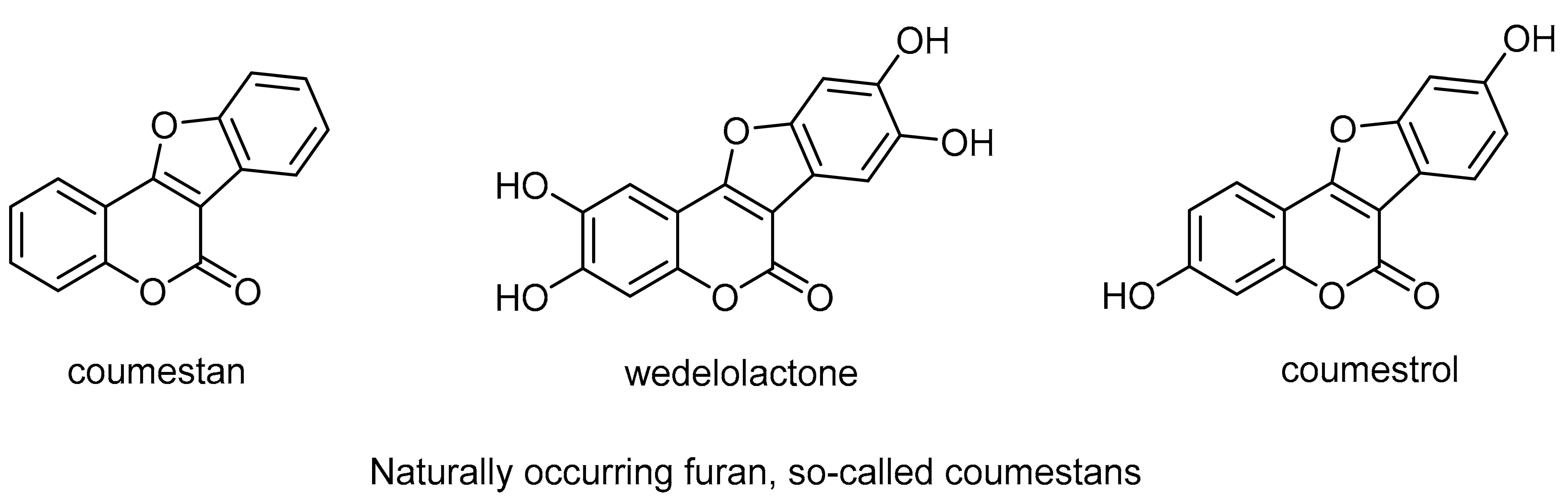
Coumarins are a family of benzopyrones (1,2-benzopyrones or 2H-[1]benzopyran-2-ones), which represent an important family of oxygen-containing heterocycles, widely distributed in nature [1,2,3,4]. Coumarins display a broad range of biological and pharmacological activities, [5,6] such as antiviral [7,8,9,10], anticancer [11,12,13], antimicrobial [14,15], and antioxidant [16,17,18] activities. On the other hand, coumarin represents an ingredient in perfumes [19], cosmetics [20], and as industrial additives [21,22]. Furthermore, coumarins play a pivotal role in science and technology as fluorescent sensors, mainly due to their interesting light-emissive characteristics, which are often responsive to the environment [23,24,25,26]. The coumarin (benzopyrane)-fused, membered aromatic heterocycles built on the α-pyrone moiety are an important scaffold. The only fused heterocycle with an α-pyrone moiety of coumarin that can be found in nature is the furan ring. One example is the naturally occurring furan 4H-furo[3,2-c]benzopyran-4-one, which provides the main core of many natural compounds of so-called coumestans. These comestans include coumestan, wedelolactone, and coumestrol. The coumestans are found in a variety of plant species that are commonly used in traditional medicine [27].
In order to enrich the limited versatility of the structures found in nature, synthesis of coumarin (benzopyrane)-fused, membered aromatic heterocycles has received considerable attention, including numerous reported routes.
2. Synthesis of Benzopyrone-Fused, Five-Membered Aromatic Heterocycles
2.1. Five-Membered Aromatic Rings with One Heteroatom
2.1.1. Furans
Furobenzopyrone (or furocoumarins) comprises an important class of coumarins found in a wide variety of plants, particularly in the carrot (Apiaceae/Umbelliferae), legume (Fabaceae), and citrus families (Rutaceae) [27]. The chemical structure of furobenzopyrone (furocoumarins) consists of a furan ring fused with coumarin. The fusion of the furan ring to the α-pyrone moiety of coumarin forms the core structure of the three most common isomers, viz. 4H-furo[2,3-c]chromen(benzopyran)-4-one, 4H-furo[3,4-c]chromen(benzopyran)-4-one, and 4H-furo[3,2-c]chromen (benzopyran)-4-one (Figure 1).
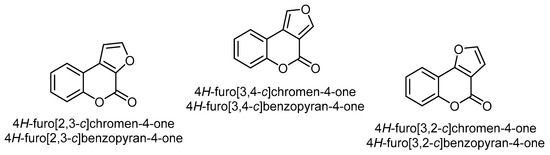
Figure 1. The three most common isomers of a furan ring fused to the α-pyrone moiety of coumarin.
4H-Furo[2,3-c]benzopyran-4-one
Furan Construction
The basic building block for the formation of 4H-furo[2,3-c]benzopyran-4-one is the 3-hydroxycoumarin (1) [28,29]. Pandya and coworkers [30] developed a method to synthesize some 4H-furo[2,3-c]benzopyran-4-ones starting with 3-hydroxycoumarin using the Nef reaction. Thus, the reaction of 3-hydroxycoumarin (1) with various 2-aryl-1-nitro ethenes 2a,b, in the presence of piperidine and methanol as a solvent, followed the Nef reaction condition and afforded a series of 1-aryl-furo[2,3-c]benzopyran-4-ones 3a,b and 1-phenyl-2-methyl-furo[2,3-c]benzopyran-4-one (4), respectively (Scheme 1). The formation of these products was explained by the reaction mechanism (Scheme 1).
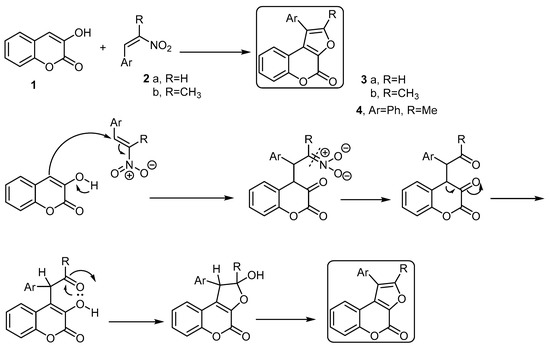
Scheme 1. The Nef reaction to synthesize furo[2,3-c]benzopyran-4-ones 3a,b and 4. Reagents and conditions: MeOH, piperidine, reflux, five outputs in 55%–61% yield.
Pyrone Construction
Dong et al., 2020 developed a novel and facile rhodium(III)-catalyzed process of sulfoxonium ylide (5) with hydroquinone (6). The carbonyl in the sulfoxonium ylide assisted the ortho-C–H functionalization of the sulfoxonium ylide, followed by intramolecular annulation with hydroquinone to afford 8-hydroxy-4H-furo[2,3-c]benzopyran-4-one (7) (Scheme 2) [31].
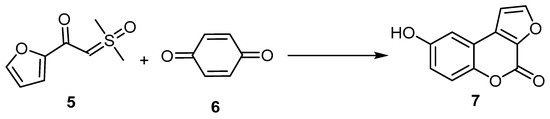
Scheme 2. Rhodium(III)-catalyzed sequential ortho-C–H oxidative arylation/cyclization of sulfoxonium ylide to afford 4H-furo[2,3-c]benzopyran-4-one (7). Reagents and conditions: [Cp*RhCl2]2 (5 mol %), AgBF4 (20 mol %), Zn(OAc)2 (0.225 mmol), AcOH (0.3 mmol), and acetone (2 mL), 12 h, in a sealed Schlenk tube under N2 at 100 °C, 25% yield.
4H-Furo[3,4-c]benzopyran-4-one
Furan and Pyrone Construction
In the literature, a large number of reports described the synthesis of 4H-furo[2,3-c] and 4H-furo[3,2-c]benzopyran-4-ones, while synthesis of the 4H-furo[3,4-c]benzopyran-4-one was reported by only one study, that of Brahmbhatt and his coworkers [32]. The first 4H-furo[3,4-c]benzopyran-4-ones (10) was synthesized by the demethylation–cyclization reaction of intermediates, 3-substituted-4-ethoxycarbonyl furans 9 (Scheme 3). For the demethylation and in situ lactonization steps, several reagents were tried, of which pyridine hydrochloride and HBr in acetic acid were found to be the most promising.
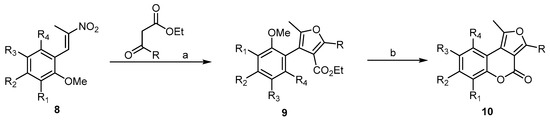
Scheme 3. Demethylation and in situ lactonization steps to prepare the first 4H-furo[3,4-c]benzopyran-4-one 10. Reagents and conditions: (a) ethyl acetoacetate or ethyl benzoylacetate, piperidine, and MeOH (the Nef reaction condition); (b) HBr, AcOH concentration, 130 °C, 4 h, 16 outputs with 50%–65% yield.
4H-Furo[3,2-c]benzopyran-4-one
A wide range of research has demonstrated that 4-hydroxycoumarin is the key compound for the synthesis of 4H-furo[3,2-c]benzopyran-4-ones, which can readily react with the C=C bond of the alkene, or the C≡C bond of the alkyne [33,34,35,36,37].
Reisch reported the condensation of 4-hydroxycoumarin (11) with 1-phenyl-2-propyn-1-ol (12) under acidic conditions (a mixture of glacial acetic and concentrated sulfuric acid) to deliver the corresponding 2-methyl-3-phenylfuro[3,2-c]benzopyran-4-one (13) (Scheme 4) [38].
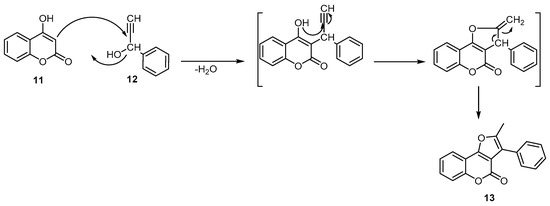
Scheme 4. Synthesis of 2-methyl-3-phenylfuro[3,2-c]benzopyran-4-one (13). Reagents and conditions: AcOH, conc. H2SO4, 110 °C, 1 h, 70% yield.
A few studies employed the aliphatic aldehydes as building blocks with 4-hydroxycoumarin (11) to synthesize 4H-furo[3,2-c]benzopyran-4-ones [25,39]. This method was ineffective as it gave a poor yield as well as a mixture of 2,3-dihydrofuran, 4H-furo[3,2-c]benzopyran-4-ones, and 4H-furo[3,2-c]benzopyran-4-ones [39]. Conversely, in the case of using the aromatic aldehyde as a building block, the 4H-furo[3,2-c]benzopyran-4-one was obtained [40]. Kadam et al. developed atom-efficient multicomponent reactions (MCRs) and step-efficient, one-pot synthesis of 3-(4-bromophenyl)-2-(cyclohexylamino)-4H-furo[3,2-c]benzopyran-4-one (16) using 4-hydroxycoumarin (11) with 4-bromobenzaldehyde (14) and cyclohexyl isocyanide (15) as an alkylene source (Scheme 5) [40].
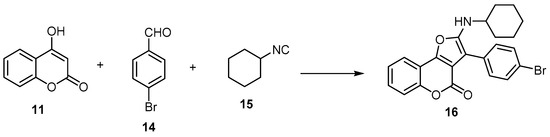
Scheme 5. Atom-efficient multicomponent reactions (MCRs) and step-efficient, one-pot synthesis of 4H-furo[3,2-c]benzopyran-4-one (16). Reagents and conditions: DMF or toluene, µw, 80 °C, 20 min, 97% yield.
4-Hydroxycoumarin derivatives have received significant attention from researchers, as these derivatives possess 1,3-dicarbonyl systems. It allows for the easy generation of α,α′-dicarbonyl radicals, which can be readily added to the C=C bond of the alkene [41]. The first example of this reaction was described in 1998, by Lee and his coworkers. They reported an efficient way to prepare 4H-furo[3,2-c]benzopyran-4-ones 19 by Ag2CO3/celite (Fetizon’s reagent)-mediated oxidative cycloaddition of 4-hydroxycoumarin 17 to olefins, such as vinyl sulfide and phenyl propenyl sulfide. The resulting dihydrofuro[3,2-c]benzopyran-4-ones 18 was treated by sodium periodate in aqueous methanol to form the corresponding sulfoxides, which, upon refluxing with pyridine in carbon tetrachloride, directly delivered the 4H-furo[3,2-c]benzopyran-4-one 19 in good yields (Scheme 6) [41].
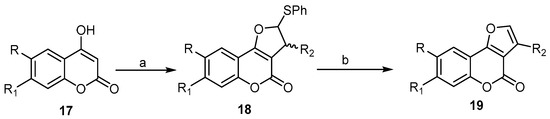
Scheme 6. A facile synthesis of 4H-furo[3,2-c]benzopyran-4-ones 19 by silver(I)/celite promoted an oxidative cycloaddition reaction. Reagents and conditions: (a) CH2=CHSPh and/or CH3CH=CHSPh, Ag2CO3/celite, acetonitrile, reflux, 3 h; (b) NaIO4, MeOH, CCl4, pyridine, Al2O3, four outputs with 71%–82% yield.
Recently, different catalytic methodologies have been developed for the synthesis of 2H-chromenes, and they are based on three main approaches: catalysis with (transition) metals, metal-free Brønsted catalysis, and Lewis acid/base catalysis, which includes examples of nonenantioselective organocatalysis and enantioselective organocatalysis [42,43,44]. Alkynes have been widely employed as building blocks for this reaction in most cases.
To date, different transition metal (Au, Pt, and Cu) catalyzed/mediated methodologies for benzopyrane synthesis have been reported [27,42,45,46]. Cheng and Hu described a one-pot cascade of an addition/cyclization/oxidation sequence using CuCl2 as the oxidant and CH3SO3H as the acid for regioselective synthesis of 2-substituted-4H-furo[3,2-c]benzopyran-4-ones 22 from the substituted 3-alkynyl-4H-benzopyran-4-one 20 (Scheme 7) [47]. This strategy included the CH3SO3H-acid-catalyzed construction of the furan ring, followed by oxidation of 21 with CuCl2 (Scheme 7) [47]. When the reaction was carried out in the presence of a catalytic amount of CuCl as a Lewis acid and atmospheric oxygen as an oxidative reagent, compound 22 was provided directly. On the other hand, the presence of 10% CuBr and an excess of CuCl2 as the oxidant afforded the corresponding 3-chloro-2-substituted- 4H-furo[3,2-c]benzopyran-4-ones 23 (Scheme 7) [48].
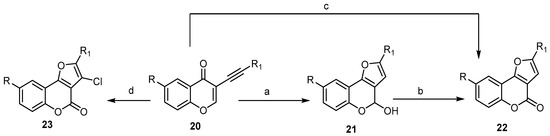
Scheme 7. Transition metal Cu catalyzed/mediated methodologies for synthesis of the 4H-furo[3,2-c]benzopyran-4-ones 22 and 23. Reagents and conditions: (a) CH3SO3H, H2O, DMF, 90 °C, 1–3 h; (b) CuCl2, 90 °C, 20 h; (c) CuCl, O2, DMF, H2O, 90 °C, 10–20 h, 10 outputs with 37%–88% yield; (d) CuBr, CuCl2, DMF, H2O, 75 °C, 10 h, 13 outputs with 45%–81% yield.
Brønsted-acid-catalyzed propargylations of several organic substrates, including 1,3-dicarbonyl compounds, with alkynols have been reported [49]. In most cases, the acid catalyst is required to promote the propargylation process efficiently. Zhou and coworkers developed a one-pot Yb(OTf)3 propargylation–cycloisomerization sequence of 4-hydroxycoumarin (11) with the propargylic alcohol (24) for the synthesis of a 2-benzyl-3- phenyl-4H-furo[3,2-c]chromen-4-one (25) skeleton using Yb(OTf)3 as a Lewis acid (Scheme 8) [50].
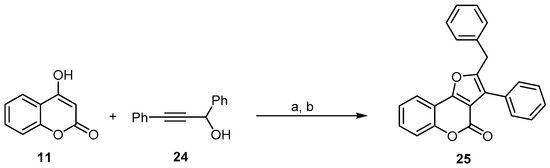
Scheme 8. One-pot synthesis of 4H-furo[3,2-c]chromen-4-one (25) using a Yb(OTf)3-catalyzed propargylation and allenylation reaction. Reagents and conditions: (a) 5 mol % Yb(OTf)3, CH3NO2, dioxane, 50 °C; (b) K2CO3, 70 °C, 37% yield.
Similarly, 4H-furo[3,2-c] benzopyran-4-one formation reactions proceeded in higher yields and in a one-pot manner, employing a catalytic system composed of the 16-electron allyl–ruthenium(II) complex [Ru(η3-2-C3H4Me)(CO)(dppf)][SbF6] (dppf=1,1′-bis(diphenylphosphino)ferrocene) and trifluoroacetic acid (TFA) in the reaction of 4-hydroxycoumarin (11), with 1-(4-methoxyphenyl)-2-propyn-1-ol (26) as an example. The 4H-furo[3,2-c]benzopyran-4-one (27) was synthesized with a 72% yield (Scheme 9) [50,51,52].
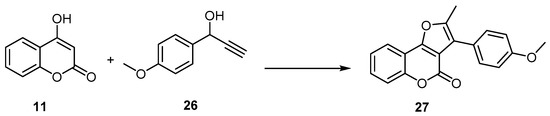
Scheme 9. The 16-electron allyl–ruthenium(II) complex in preparation of 4H-furo[3,2-c]benzopyran-4-one (27). Reagents and conditions: 16-electron allyl–ruthenium(II) complex [Ru(η3-2-C3H4Me)(CO)(dppf)][SbF6] (5 mol %), trifluoroacetic acid (TFA) (50 mol %), THF, 75 °C, 5 h, 72% yield.
Extensive work has been done to investigate the utility of an aryl alkynyl ether as a furan substrate, instead of arylalkynol, in the synthesis of 4H-furo[3,2-c]benzopyran-4-one [29,35]. The treatment of 3-iodo-4-methoxycoumarin (28) with phenylacetylene by means of sequential Sonogashira C–C coupling conditions resulted in a high-yield formation of the 4H-furo[3,2-c]benzopyran-4-one (30) (Scheme 10) [53]. In this reaction, the triethylamine was used as a base to induce the SN2-type demethylation of the Sonogashira coupling product, followed by an intramolecular attack of the enolate onto the cuprohalide π-complex of the triple bond (Scheme 10).
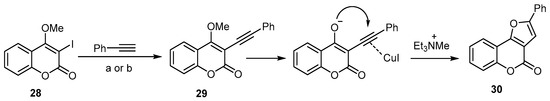
Scheme 10. Et3N-induced demethylation–annulation of an aryl alkynyl ether in the synthesis of 4H-furo[3,2-c]benzopyran-4-one (30). Reagents and conditions: (a) alkyne (3 equiv.), 8 mol % PdCl2(PPh3)2, 8 mol % CuI, Et3N/DMF, 80 °C, 48 h, 82% yield; (b) alkyne (3 equiv.), 8 mol % PdCl2(PPh3)2, 8 mol % CuI, Et3N/MeCN, 60 °C, 15 h, 70% yield.
As a follow-up to this type of reaction, a novel and rapid assembly of an interesting class of 4H-furo[3,2-c]benzopyran-4-ones, 33, was successfully achieved using a one-pot sequential coupling/cyclization strategy with 3-bromo-4-acetoxycoumarins 31 and dialkynlzincs 32 prepared in situ as reactive acetylides in transition-metal-catalyzed crosscoupling. The cascade transformation relies on palladium/copper-catalyzed alkynylation and intramolecular hydroalkoxylation (Scheme 11) [54].
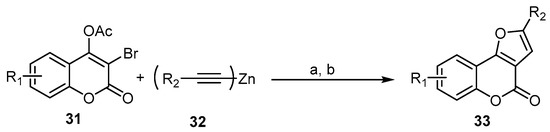
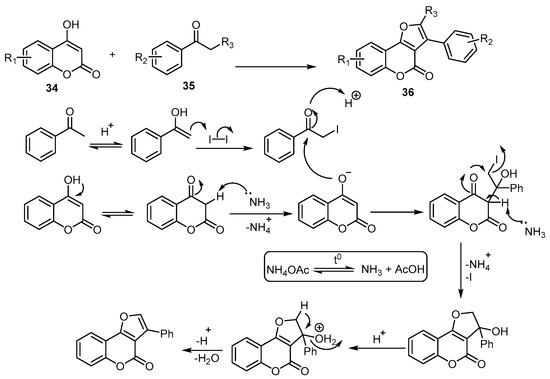
A transition-metal-free approach was developed to achieve 4-H-furo[3,2-c]benzopyran-4-ones via an iodine-promoted one-pot cyclization between 4-hydroxycoumarins 34 and acetophenones 35. The transformation spontaneously proceeded to produce (36) in the presence of NH4OAc. The possible reaction mechanism suggested for the iodine-promoted one-pot cyclization is depicted (Scheme 12) [55].
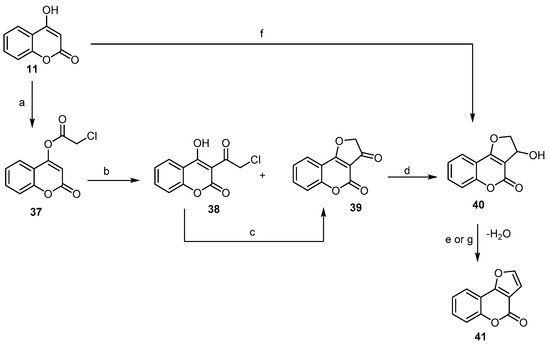
Additionally, Traven et al. [56] provided a new short way for the synthesis of 4H-furo- [3,2-c]benzopyran-4-one, employing the Fries rearrangement of 4-chloroacetoxycoumarin (37) to yield two products, namely 3-chloroacetyl4-hydroxycoumarin (38) and dihydrofuro[2,3-c]coumarin-3-one (39), in the ratio of 2:1. Compound (38), which underwent cyclization, led to the formation of (39). The latter, under reduction and dehydration conditions, afforded 4H-furo[3,2-c]chromen-4-one (41) (Scheme 13). A closely related reaction that allowed for the preparation of (41) was developed by Majumdar and Bhattacharyya [57], following a similar procedure but using chloroacetaldehyde instead of chloroactylchloride in the presence of aqueous potassium carbonate to give 3-hydroxy-2,3- dihydrofuro[3,2-c]benzopyran-4-one (40), which upon treatment with aqueous hydrochloric acid provided 4H-furo[3,2-c]benzopyran-4-one (41) with 72% yield (Scheme 13).
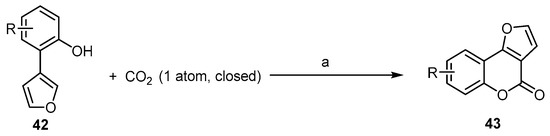
Recently, much effort has been devoted to the development of oxidative intramolecular C–O bond-forming cyclization reactions for the synthesis of bioactive benzopyranones. These methods are limited to being used with arenes building blocks [58,59,60]. Fu et al. reported a ligand-enabled, site-selective carboxylation of 2-(furan-3-yl)phenols 42 under the atmospheric pressure of CO2. It was performed through an Rh(ii)-catalyzed C–H bond activation, assisted by the ligand chelation of the phenolic hydroxyl group to afford 4H-furo[3,2-c]benzopyran-4-ones 43 (Scheme 14) [61]. This reaction indicates the role of phosphine ligands in combination with Rh2(OAc)4 in promoting the reactivity and the selectivity during C–H carboxylation. The right choice of a suitable basic catalyst is an additional critical point.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26020483
