Lignin, the term commonly used in literature, represents a group of heterogeneous aromatic compounds of plant origin.
- lignin
- technical lignin
- technology
- lignin structure
- lignin nanoparticle
1. Introduction
Technical lignin refers to the “native-lignin” or “proto-lignin” derivative obtained as the result of the delignification process of the lignocellulosic-biomass [1]. The technical lignin structure can vary with the process and chemical reactions utilized in the biomass/starting material treatment [2]. Traditionally, technical lignin has been obtained as a by-product of paper mills wherein the revenue is primarily generated by the cellulose-based products, and therefore every process has been optimized to maximize the qualitative and quantitative yield of the cellulose [3]. The recent progress in technology and the arrival of “lignin-centric biorefinery” or “lignin-first biorefinery” is a welcomed addition to the biomass treatment strategies. It has renewed the interest in lignin from “valorization” to “application” approaches [4][5][6][7]. It has also provided an impetus to the search for structurally homogeneous lignin that can be easily applied in efficient and scalable technology for lignin-based reagents production [8]. The relatively new addition in lignin-based clean ( biocompatible and non-toxic) products include nanomaterials and those used in biomedical applications [9][10][11]. As presented in Figure 1, a multi-fold rise in the number of patents registered in the Scopus database in the period of the last 20 years (2000–2019) can be seen. The maximum number of patents were registered in the United States Patent and Trademark Office (45,268), followed by the Japan Patent Office (16,547), the World Intellectual property organization (10,657), the European Patent office (8180) and the United Kingdom Intellectual Property office (332). These results were obtained from the Scopus database with the keyword “lignin”. It reflects the commercial relevance of lignin-based research and development of technology for its high–value utilization.
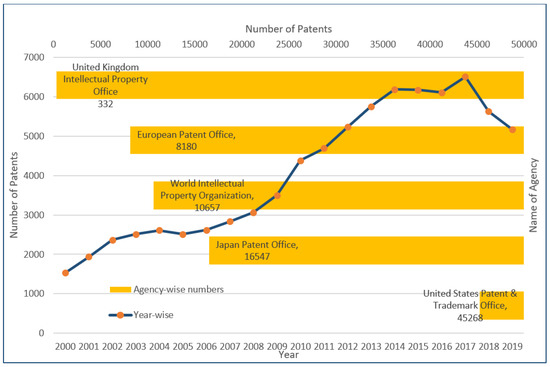
Figure 1. Number of patents registered in different agencies and the number of total patents in the duration of 2000–2019 (Results were obtained from the Scopus database using the keyword “lignin”).
Technical lignin is derived mainly from lignocellulosic biomass, and therefore its structure directly depends on the source and method of extraction. Once the lignin (protolignin/native lignin) leaves the cell wall (extracted), its structure completely changed; however, monomers can still be identified. The lignin monomers include p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol, bearing p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) units, as represented in Figure 2. The source-dependent variation in lignin can be observed in herbaceous crop-based (rich in H units), gymnosperm/softwood (lacking S units), and angiosperm/hardwood (rich in G and S) lignin [1][12]. The variation in monomers composition is supported by variation in nature and the extent of chemical bonding. Different bonds observed in lignin, along with their numbering system, are presented in Figure 3. The β-O-4 linkage represents the most dominant linkage of all three (softwood, hardwood, and grasses) types of sources; however, their comparative content follows the order grasses > hardwood > softwood. The two diastereomers, the erythro and threo forms of β-O-4 linkage, can be found in nature. The softwood lignin has approximately the same amount of both forms; the erythro form is prevalent in hardwood lignin. The quantification of the two forms (ratio and total amount) of these two forms can be done by ozonation that leads to the formation erythronic (from erythro form) and threonic (from threo form) acid, respectively [13]. The β-O-4 linkage is also the most easily affected bonding in the lignin by different treatment methods. Despite the relatively lesser number of β-O-4 linkage, the softwood lignin is primarily composed of coniferyl alcohol, highly condensed and a higher number of C-C bonds, 5′ linkages, β-β, and β-5 bonds, cross-linking, and branching. These properties make softwood challenging to degrade and relatively more resistant to chemical treatments. Another aromatic material (not a lignin derivative) found in pulp analysis is termed pseudolignin. It consists of aliphatic and aromatic groups (methoxyl, carboxyl, and carbonyl groups) of non-lignin origin. Pseudolignin has also been observed in pulp obtained by steam explosion and dilute acid hydrolysis. [14][15]. It has been suggested that its formation is favored by low pH, high temperature, longer treatment time, and acid strength. The mechanism of pesudolignin formation has been suggested as repolymerisation of decomposition products of the sugars [16][17]. However, the pseudolignin formation is a different aspect of biomass delignification, and a detailed discussion on it is out of the scope of this manuscript.
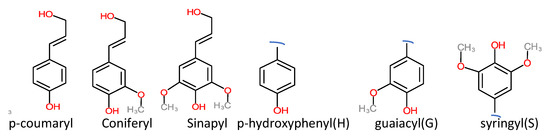
Figure 2. Common monomers of lignin.
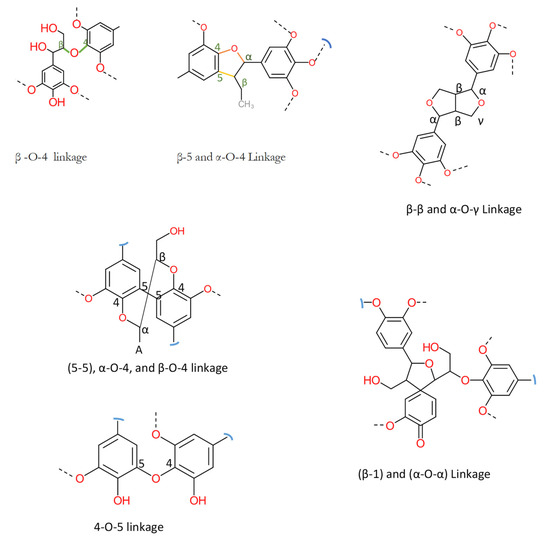
Figure 3. Common linkages found in lignin [18].
In the case of technical lignin, the generalized information about lignin is not sufficient. The “technical lignin” specific data is needed to manipulate the material at molecular/nanoscale material for its utilization in high-value applications. Unfortunately, unanimously accepted data about technical lignin molecular characteristics is still missing [13][19]. It is due to the fact that even for the same method such as nuclear magnetic resonance (NMR) and size exclusion chromatography (SEC), the data varies depending on the chosen parameters. Several excellent reviews on lignin properties and applications have been published recently [20][21][22][23][24][25]. However, this review builds upon the fact that papermill-based technical lignin is still a standard and abundantly available raw material; therefore, successful technology must be built on its coarse but scalable properties. Different technical lignin (commercially available), their characteristics, and reported studies have been critically reviewed to develop a coarse level of generalized understanding. The recently reported (last 3 years) technical lignin nanoparticle and industry-specific applications have been studied along with marketed products. The gap between the lab-scale suggested application and marketed formulations has been used to underline lignin utilization challenges. Additionally, the future potential and challenges for technical lignin in the high-value applications have been covered for future applications.
2. Classification of Technical Lignin
The overall classification of lignin generally follows the criteria of the delignification/biomass treatment approach. Especially for technical lignin, a number of variations in pretreatment and/or lignin precipitation methodologies can be classed in the same categories. These classes also represent the changes in delignification and precipitation methods with time, leading to improved technology and, therefore, the final product. Several types of lignin can be obtained by the same broader class of treatment, such as Kraft lignin. Every delignification approach modifies the structure of lignin in its specific way, and it can be observed in the generalized representative structure of lignin in Figure 4. In addition to the generalized structure, treatment-based variation in nature and extent of C-C bond cleavage, degree of condensation, molecular weight, and quantitative and qualitative variations in functional groups of the obtained technical lignin are also important. These also directly affect the potential applications of technical lignin. For example, Kraft lignin contains covalently bonded sulfur species that constitute major impurities that limit and direct the further applications and valorization approaches (sulfur is known as poison for many metal-containing catalysts used for depolymerization). On the other hand, lignosulfonate has the sulfonate group on the aromatic ring that endows water-solubility. Additionally, their colloidal, surfactant and adhesive properties are due to the high density of functional groups. It is due to specific bond breakage caused by particular solvent-biomass interaction and the underlying mechanism. These aspects are discussed in detail in the following section.
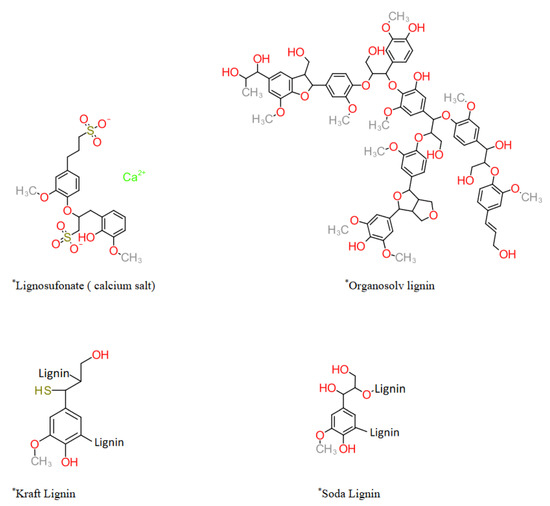
Figure 4. Common technical lignins (* simplified and representative structures).
This entry is adapted from the peer-reviewed paper 10.3390/ijms22010063
References
- Achyuthan, K.E.; Achyuthan, A.M.; Adams, P.D.; Dirk, S.M.; Harper, J.C.; Simmons, B.A.; Singh, A.K. Supramolecular Self-Assembled Chaos: Polyphenolic Lignin’s Barrier to Cost-Effective Lignocellulosic Biofuels. Molecules 2010, 15, 8641–8688.
- Kopsahelis, N.; Agouridis, N.; Bekatorou, A.; Kanellaki, M. Comparative Study of Spent Grains and Delignified Spent Grains as Yeast Supports for Alcohol Production from Molasses. Bioresour. Technol. 2007, 98, 1440–1447.
- Ashori, A. Nonwood Fibers—A Potential Source of Raw Material in Papermaking. Polym. Plast. Technol. Eng. 2006, 45, 1133–1136.
- Argyropoulos, D.S. Quantitative Phosphorus-31 NMR Analysis of Six Soluble Lignins. J. Wood Chem. Technol. 1994, 14, 65–82.
- Balakshin, M.Y.; Berlin, A.; DelliColli, H.T.; Grunert, C.A.N.J.; Gutman, V.M.; Ortiz, D.; Pye, E.K. Derivatives of Native Lignin. U.S. Patent 8,445,562, 21 May 2013.
- Dawy, M.; Shabaka, A.A.; Nada, A.M.A. Molecular Structure and Dielectric Properties of Some Treated Lignins. Polym. Degrad. Stab. 1998, 62, 455–462.
- Mansfield, S.D. Solutions for Dissolution—Engineering Cell Walls for Deconstruction. Curr. Opin. Biotechnol. 2009, 20, 286–294.
- Ahvazi, B.; Cloutier, É.; Wojciechowicz, O.; Ngo, T.-D. Lignin Profiling: A Guide for Selecting Appropriate Lignins as Precursors in Biomaterials Development. ACS Sustain. Chem. Eng. 2016, 4, 5090–5105.
- Siddiqui, L.; Bag, J.; Mittal, D.; Leekha, A.; Mishra, H.; Mishra, M.; Verma, A.K.; Mishra, P.K.; Ekielski, A.; Iqbal, Z. Assessing the Potential of Lignin Nanoparticles as Drug Carrier: Synthesis, Cytotoxicity and Genotoxicity Studies. Int. J. Biol. Macromol. 2020, 152, 786–802.
- Siddiqui, L.; Mishra, H.; Mishra, P.K.; Iqbal, Z.; Talegaonkar, S. Novel 4-in-1 Strategy to Combat Colon Cancer, Drug Resistance and Cancer Relapse Utilizing Functionalized Bioinspiring Lignin Nanoparticle. Med. Hypotheses 2018, 121, 10–14.
- Mishra, P.K.; Ekielski, A. A Simple Method to Synthesize Lignin Nanoparticles. Colloids Interfaces 2019, 3, 52.
- Poletto, M.; Zattera, A.J. Materials Produced from Plant Biomass: Part III: Degradation Kinetics and Hydrogen Bonding in Lignin. Mater. Res. 2013, 16, 1065–1070.
- Santos, R.B.; Capanema, E.A.; Balakshin, M.Y.; Chang, H.; Jameel, H. Lignin Structural Variation in Hardwood Species. J. Agric. Food Chem. 2012, 60, 4923–4930.
- Negro, M.; Manzanares, P.; Oliva, J.; Ballesteros, I.; Ballesteros, M. Changes in Various Physical/Chemical Parameters of Pinus Pinaster Wood after Steam Explosion Pretreatment. Biomass Bioenergy 2003, 25, 301–308.
- Poulomi, S.; Dong Ho, K.; Seokwon, J.; Arthur, R. Pseudo-Lignin and Pretreatment Chemistry. Energy Environ. Sci. 2011, 4, 1306–1310.
- Hu, F.; Jung, S.; Ragauskas, A. Pseudo-Lignin Formation and Its Impact on Enzymatic Hydrolysis. Bioresour. Technol. 2012, 117, 7–12.
- Kumar, R.; Hu, F.; Sannigrahi, P.; Jung, S.; Ragauskas, A.J.; Wyman, C.E. Carbohydrate Derived-pseudo-lignin Can Retard Cellulose Biological Conversion. Biotechnol. Bioeng. 2013, 110, 737–753.
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215.
- Bouxin, F.P.; McVeigh, A.; Tran, F.; Westwood, N.J.; Jarvis, M.C.; Jackson, S.D. Catalytic Depolymerisation of Isolated Lignins to Fine Chemicals Using a Pt/Alumina Catalyst: Part 1—Impact of the Lignin Structure. Green Chem. 2015, 17, 1235–1242.
- Hubbe, M.A.; Alén, R.; Paleologou, M.; Kannangara, M.; Kihlman, J. Lignin Recovery from Spent Alkaline Pulping Liquors Using Acidification, Membrane Separation, and Related Processing Steps: A Review. Bioresources 2019, 14, 2300–2351.
- Norgren, M.; Edlund, H. Lignin: Recent Advances and Emerging Applications. Curr. Opin. Colloid Interface Sci. 2014, 19, 409–416.
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092.
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and Chemical Modifications of Lignin: Towards Lignin-Based Nanomaterials for Biomedical Applications. Prog. Mater. Sci. 2018, 93, 233–269.
- Mishra, P.K.; Ekielski, A. The Self-Assembly of Lignin and Its Application in Nanoparticle Synthesis: A Short Review. Nanomaterials 2019, 9, 243.
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards Lignin-Based Functional Materials in a Sustainable World. Green Chem. 2016, 18, 1175–1200.
