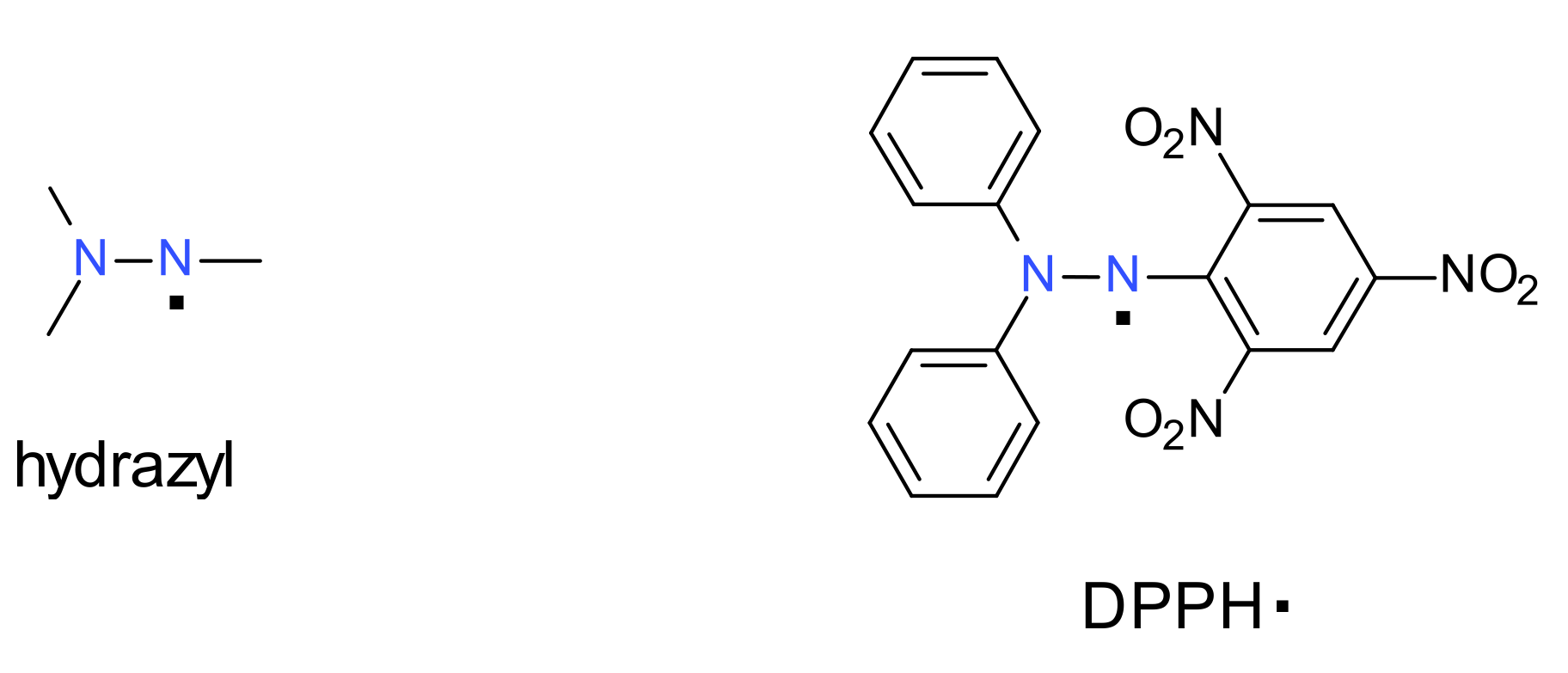
DPPH· is 2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl stable free radical. It was discovered in 1922.
- hydrazyl
- free radical
- EPR
- ESR
- spin-trapping
- diradical
1. Introduction
The chemistry and physics of stable or persistent free radicals are well represented by several books [1,2,3], two of which place a greater emphasis on their organic reactions, the first one being published in 1968 [4] and the second one in 2010 [5]. However, as mentioned in the literature [6], in the most recent there is a notable absence of hydrazyl (and also aminyl) free radicals. Many of free radicals derived from the most encountered stable hydrazyl (DPPH, Figure 1).
1.1. Free Radicals
A free radical is a chemical entity that contains an unpaired electron (free electron) that possess a quantum-mechanical property called spin. Such an entity typically has a high reactivity due to its open-shell structure. However, nowadays there are a lot of such known compounds that are stable under usual laboratory conditions (room temperature and presence of air) [3,4,5]. Their high stability is a sum of several structural characteristics, the most important being steric hindrance and conjugation. The push–pull effect that contributes to the greater stability of several classes of such free radicals was postulated in the 1960s [7].
1.2. Hydrazyls
A hydrazyl free radical contains the chemical moiety denoted in Figure 1 Left, where the dots represents the unpaired electron. The most known hydrazyl free radical is 2,2-diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl, usually encountered as DPPH· (the 2,4,6-trinitrophenyl fragment is also frequently named picryl).
The history of hydrazyl free radicals starts about 100 years ago, when Goldschmidt firstly observed that oxidation of triphenylhydrazine led to an intense blue color that fades rapidly [8]. Between many congeners of triphenylhydrazine, he found that the oxidation of 2,2-diphenyl-1-picrylhydrazine, a yellow compound, also gives an intense violet solution, but in this particular case the color is stable. In this way, Goldschmidt isolated in high yields the first stable hydrazyl free radical [9]. It was found that this free radical does not dimerize or react with oxygen and is stable either in solution or in solid state. However, it is still a reactive compound, active in many types of reactions, as will later be shown.
1.3. DPPH· Free Radical
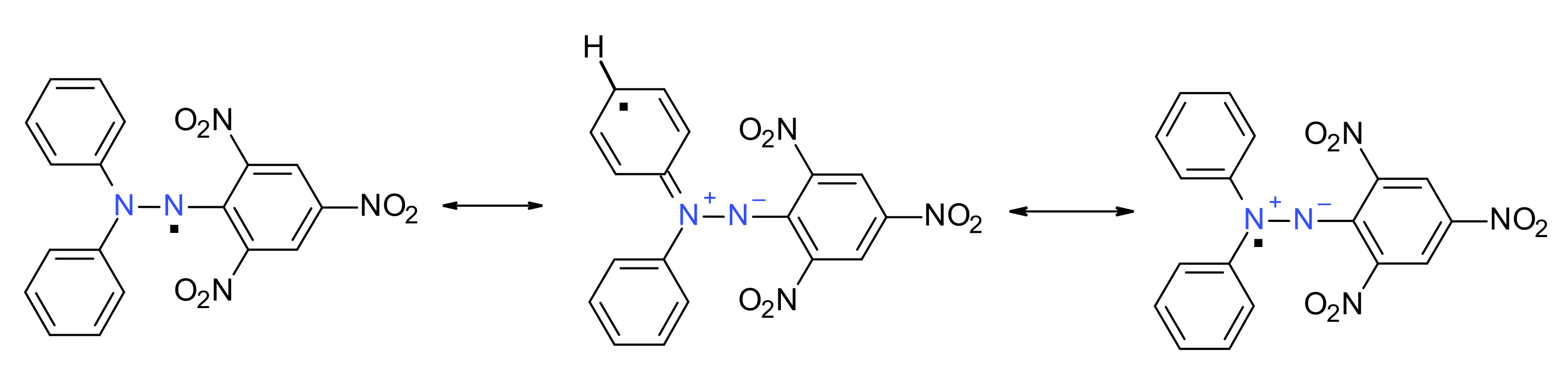
The stability of such hydrazyl free radicals may be easily evidenced in the case of DPPH·. As mentioned before, steric hindrance and conjugation play a dominant role. The push–pull effect (as picryl moiety plays the role of the electron-acceptor part of the molecule and the diphenylamino moiety plays the role of the electron-donor part of the molecule) is clearly evidenced by the resonance structures (Figure 2) and is in accordance with Linnet theory [10]. Another important aspect that is worth remembering is the dipole moment of DPPH· (4.88 D), higher than DPPH-H (3.59 D), emphasizing the importance of the polar resonance structures [11]. The N-N bond also has a higher bond order, as was directly demonstrated by the X-ray structure [12]. Due to its extreme stability, DPPH· is nowadays used as a standard in Electron Spin Resonance (ESR) spectroscopy [3,13].
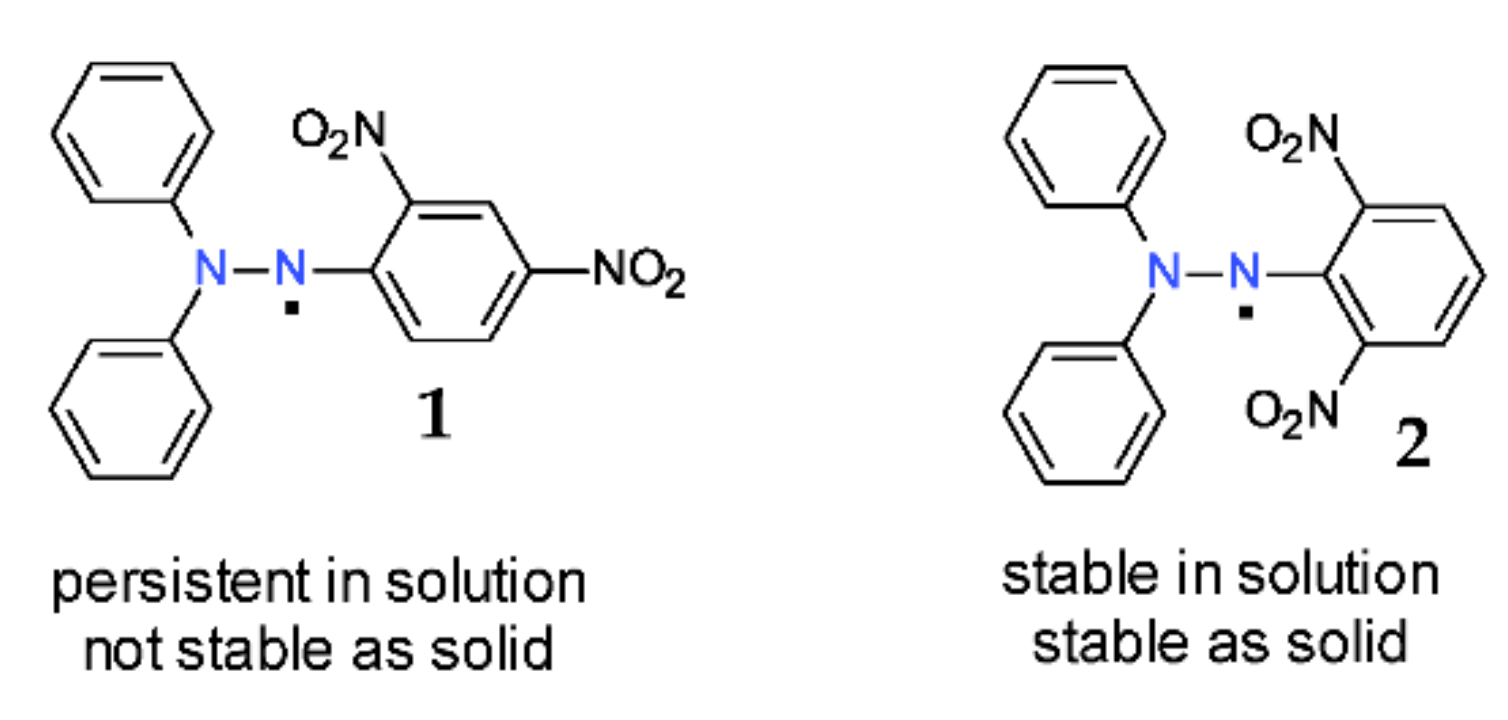
The extreme stability of the DPPH· free radical is easily demonstrated by the comparison with its congeners, 2,2-diphenyl-1-(2,4-dinitrophenyl) hydrazyl 1 and 2,2-diphenyl-1-(2,6-dinitrophenyl)-hydrazyl 2 free radicals (Figure 3). The most important nitro groups are situated in the ortho-position with regard to the nitrogen atom; thus, between the two 2,4-dinitro and 2,6-dinitro isomers 1 and 2 (Figure 3), there is a huge difference in terms of their stability [14,15]. Accordingly, isomer 1 (2,4-dinitro) can exist only in solution (with a half-life of about 90 h) and not in solid state, while isomer 2 (2,6-dinitro) is stable both in solution and in solid state [16]. As a consequence, most of the free radicals of such type that contain any other group in position 6 (para-) are stable [4].
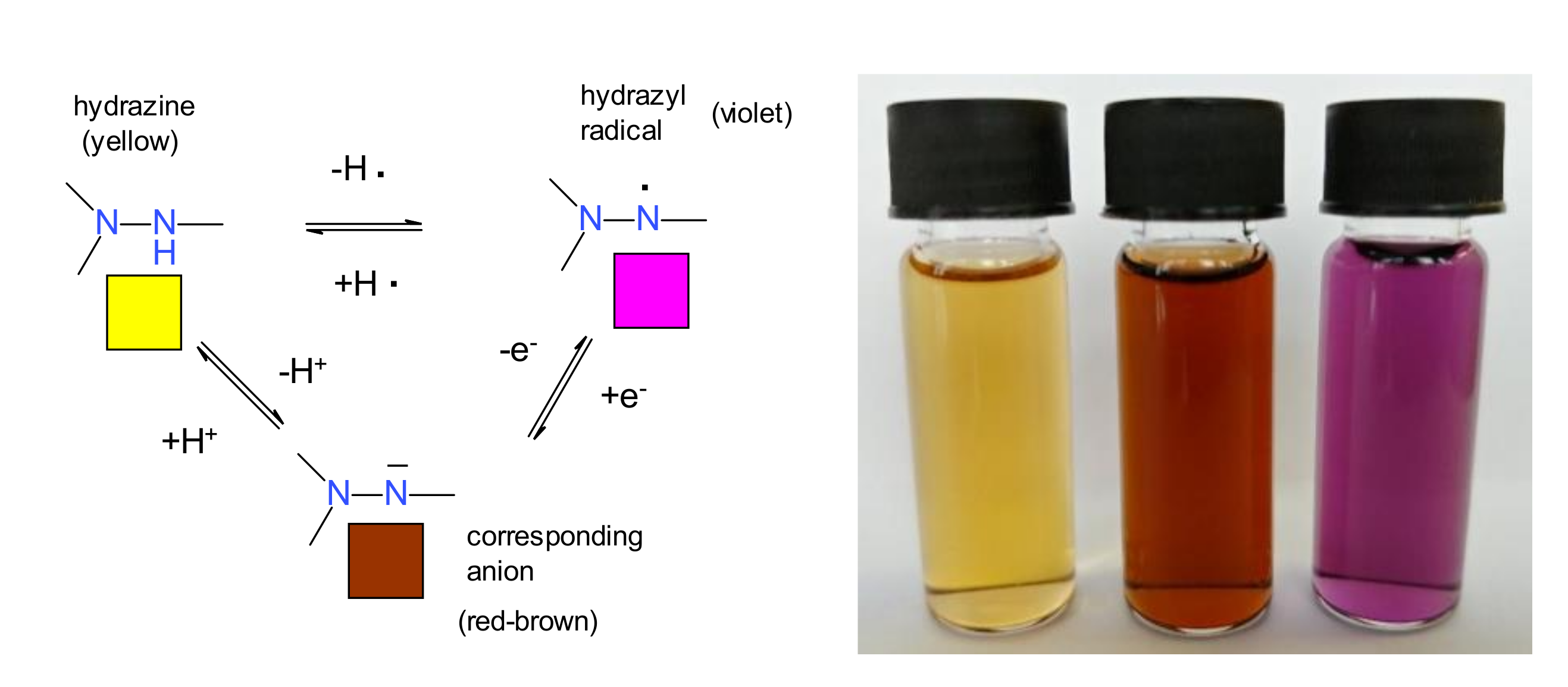
The hydrogen atom bound to the hydrazine group cannot only to be removed by oxidation, leading to the hydrazyl free radical, but also by a base, because it has an acidic character. Most of the hydrazyl free radicals have a violet color, while the parent hydrazines are yellow; the corresponding anion is usually red-brown, but it is possible also to be green, depending on the substituents. All these acid-base or redox processes are reversible, as shown in Figure 4 [17].
2. Application of Hydrazyls
2.1. Acid-Base and Redox Processes
It was shown before that mainly the p-substituents on the phenyl ring has the most influence on the oxidation capacity of the DPPH· congeners, as well as on their acidity (for parent hydrazines). Concerted electron–proton transfer may occur [60]. For example, each supplementary p-nitro group increases the oxidation potential of the free radicals by 0.1–0.2 V, while, for parent hydrazines, each group lowers the pKa value by about 1 unit. This means that hydrazyl free radicals become stronger oxidants, whereas hydrazines become stronger acids. Table 2 shows these values.
Table 2. λmax (nm), pKa, Eox (V) and bond dissociation energy (BDE) values (kcal/mol) for selected compounds [17,27,39,46,56,61].
| Compound | λradical | λanion | λhydrazine | pKa | Eox | BDE |
|---|---|---|---|---|---|---|
| DPPH | 518 | 424 | 322 | 8.54 | 0.30 | 75.3 |
| 1 | 512 | 383 | 337 | 11.3 | 0.13 | 74.5 |
| 9 | 500 | 505 | 352 | 7.35 | 0.49 | - |
| 10 | 500 | 485 | 352 | 6.49 | 0.60 | - |
| 12 | 524 | 413 | 353 | 8.20 | 0.34 | 82 |
| 13 | 506 | 441 | 363 | 10.7 | 0.25 | 76.4 |
| 23 | 500 | 365 | 346 | 11.1 | 0.24 | 76.3 |
| 25 | 524 | 655 | 324 | 12.5 | 0.87 | 76.81 |
| 26 | 517 | 495 | 330 | 11.6 | 1.17 | 76.96 |
| 27 | 517 | 492 | 332 | 10.9 | 1.23 | 70.67 |
| 28 | 486 | 620 | 392 | 7.82 | 0.173 | 75.45 |
| 29 | 507 | 645 | 404 | 8.07 | 0.107 | 75.08 |
| 30 | 510 | 654 | 482 | 8.33 | 0.130 | 75.30 |
All these redox or acid-base processes can be followed by color change [62]. Table 2 also compiles the wavelength values where hydrazyl free radicals, their parent hydrazines or the corresponding anions have the maximum absorption. One important aspect that can be easily evaluated, knowing the pKa and Eox values, is the bond dissociation energy- BDE for the N-H bond. This can be evaluated following the Equation (1) [63].
As a remark on Table 2, it can be concluded that all the BDE values are closer to 75 kcal/mol and this is due to the fact that values of pKa and Eox compensate each other (DPPH has the literature BDE value ~75–80 kcal/mol [64]; a revised and accurate value was reported as 79 kcal/mol [65]). These BDE values can be regarded as an important tool in elucidation of the H-abstraction mechanism of DPPH congeners. As mentioned by Bordweel [63], most substituents on DPPH congeners will play a dual role in affecting the stability of such radicals, inducing both stabilization by the delocalizing property and at the same time destabilization by the electron-withdrawing ability. Recently, an electrografting method employing diazonium chemistry was used for the isolation of the aryl radical/DPPH coupling product [66].
2.2. Generators of Short-Lived Radicals
The oxidant capacity of hydrazyl free radicals can be used in the generation of short-lived radicals. It was stated before that DPPH· can abstract one electron from an anion X− yielding the radical X. (Figure 17). In a similar way, DPPH· can abstract one hydrogen atom from other compounds, yielding again unstable free radicals (for example, from phenols, Figure 10). Such processes were used in the generation of several radicals of different types, O-, N-, S, C-, or P-centered [67]. These short-lived radicals are best evidenced by the ESR spin-trapping technique that uses a diamagnetic unsaturated compound that reacts with the short-lived free radical forming a persistent nitroxide, with a half-life of minutes-hours. These compounds are called spin-traps, and most of them are nitrones or nitroso derivatives [68].
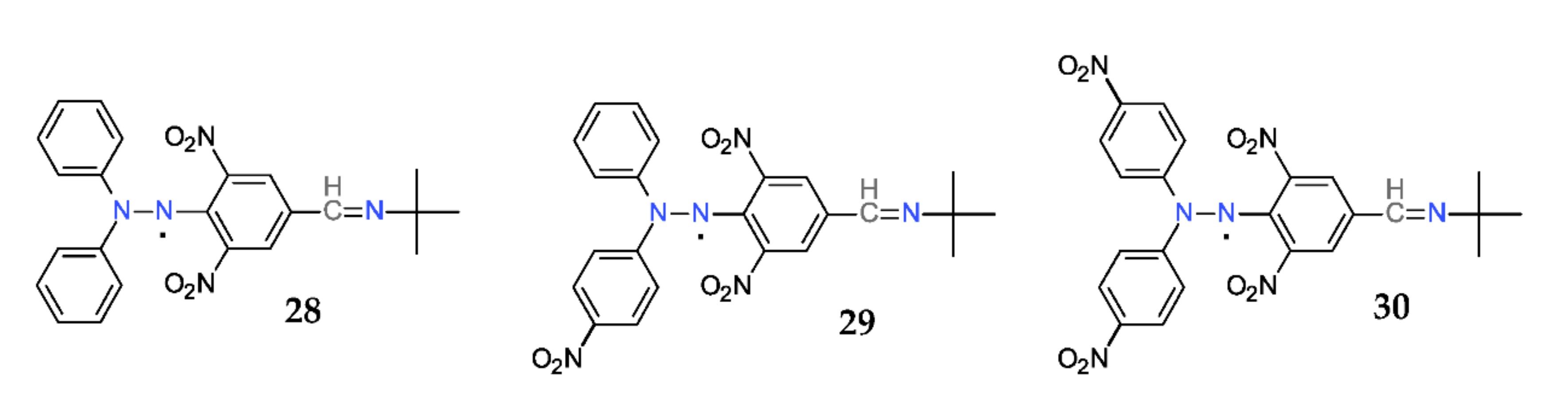
Because the spin-trapping process requires both the presence of a short-lived free radical and a spin-trap, and also because it is a necessary a system that generates those short-lived free radicals, a step-forwarding idea was to design hybrid molecules that contain a hydrazyl and a nitrone moiety covalently linked. Such compounds (28–30) are shown in Figure 18 [61].
Figure 18. Hybrid hydrazyl-nitrones.
These compounds have the great advantage of finding direct applications as sensors or probes in ESR spectroscopy, as simultaneous generators and traps for short-lived radicals. The so-called DPPH method for the total antioxidant capacity measurement is one of the most used [69,70].
3. Conclusions and Outlook
Besides all these general and particular aspects, hydrazyl free radicals are still a particular domain in chemistry that contains unexplored fields. Recently, DPPH· found applications in very different areas, such as catalysis [71,72]; it may also may find similar applications in organic battery technologies [73,74]. Another possibility of employing hydrazyl radicals and their congeners is related to the structure of betaines 15 and 20 to exist as diradicals (similar to Thiele, Tschitschibabin or Muller hydrocarbons) [75,76,77]. Although most recent literature data is about the use of DPPH free radical as scavenger in antioxidant measurements [69,70,78], there is a lot of room to develop new compounds and processes involving such stable open-shell structures, as their multifunctionality [61,79] provides real working opportunities. Along with the well-known class of nitroxide free radicals [80], hydrazyl radicals stabilized by the captodative effect [81] will fulfill and complete novel and important possibilities. Future work will bring out more interesting and unexpected results.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22041545