Human amnion-derived stem cells (hADSCs) are referred as the cells of possessing the abilities of self-renew and differentiation, which are isolated from human amnion and include human amniotic mesenchymal stem cells (hAMSCs) and human amniotic epithelial stem cells (hAESCs).
- amniotic membrane
- human amniotic stem cells
- human amniotic mesenchymal stem cells
- human amniotic epithelial stem cells
- regenerative medicine
1. Introduction
Stem cells, defined by dual hallmark features of self-renewal and differentiation potential, can be derived from embryonic and adult tissues. Stem cells are classified to pluripotent stem cells (PSCs), multipotent stem cells, and unipotent stem cells based on their developmental potency[1]. PSCs are able to form all tissues/cells with distinct functional properties which depend upon the derived and cultured conditions[2]. Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) are the two most common types of PSCs[3]. Multipotent stem cells, such as hematopoietic stem cells, are restricted to generating the mature cell types of their tissue of origin and they exist in the resting state under normal physiologic circumstances and are activated when these tissues receive nociceptive stimulation[4]. Unipotent stem cells possess the capability of self-renewal and limited differentiation potential and only produce a single cell type. The most typical unipotent stem cells are spermatogonial stem cells, which can only differentiate into sperm[5]. In the early embryo, PSCs represent progenitors for all tissues while later in the development, tissue-restricted adult stem cells (ASCs), including multipotent stem cells and unipotent stem cells, give rise to cells with highly specialized functions. Unlike ESCs and iPSCs, tissue-restricted ASCs are limited in their potency to the cell types of the tissue in which they reside[6]. ASCs derived from different tissues showed an attractive application clinically due to their abilities to differentiate into a certain type or a designated type of specific cells and have little risk of tumorigenicity and immune rejection[6][7][8][9]. When tissues and organs are damaged, sufficient tissue-ASCs are essential in maintaining tissue regeneration and functional integrity.
Although researchers have made endless efforts to improve the technologies of ESC and iPSCs, there still are two prominent hardship, tumorigenicity and low survival rate of transplanted cells/tissues, leading to enormous challenges in clinical application[10][11]. In addition, the differentiation of ESCs and iPSCs to different cells is a stepwise process that is involved in a combination of transcription factors. During the in vitro inducing process, the cells generated from transdifferentiation of ESCs or iPSCs may not possess biological function. In addition, ASCs have also certain limitations, such as the limited pluripotency, the reduced numbers with aging and the ability of the restricted expansion in vitro. Some studies have showed that ASCs were not intrinsically immunoprivileged, and under appropriate conditions, allogeneic ASCs might also induce immune rejection of an allogeneic graft[12][13]. In addition, studies also showed that the gradual accumulation of genetic mutations in human ASCs during life were able to be transmitted to daughter cells and initiate tumorigenesis[14][15].
Human amnion-derived stem cells (hADSCs) including human amniotic epithelial stem cells (hAESCs) and human amniotic mesenchymal stem cells (hAMSCs) have the great advantages over other stem cells such as renewal, multi-differentiation potential, no-tumorigenicity, low/no immunogenicity, no ethical or legal concerns and their potent paracrine effects, especially immunomodulatory effects, making them have a promising source of stem cells for cell therapy in various diseases[16][17][18].
2. Characteristics of Human Amnion-Derived Stem Cells
2.1. Advantages of hADSCs over Other Stem Cells
Placenta is composed of the amnion member, chorionic plate, decidua basalis, chorionic villi, cotyledons/interuillous space, and placental septa (Figure 1A)[8][19]. Among these placental components, the amniotic membrane serves as a suitable raw materials for cell-based therapy due to the large number of cells [20]. The amniotic membrane is a transparent, smooth, avascular and single-layered thin membrane (about 100 μm) composed of epithelium and mesenchyme. The membrane covers the fetus and holds the amniotic fluid[21]. Generally, amnion membrane has five layers including epithelium, basement membrane, compact layer, fibroblast layer and spongy layer (Figure 1B)[22]. Epiblast-derived hAMSCs and hypoblast-derived hAESCs are two primary stem cell types in amniotic membrane which are responsible for the production of extracellular matrix (ECM), different cytokines and growth factors[8]. hAESCs come from the innermost layer of amnion which directly contact with amniotic fluid and fetus, whereas hAMSCs are scattered in the membrane[18]. Isolation protocols have been extensively described for both hAESCs and hAMSCs. Briefly, the amniotic membranes were treated with trypsin-EDTA for 45–60 min at 37 °C to release hAESCs[23]. Then, the remaining amniotic membranes were digested with Collagenase IV on a rotator 40 min at 37 °C to isolate hAMSCs[24].
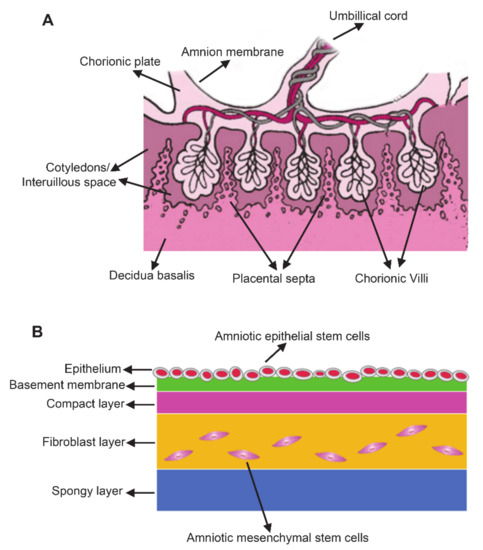
Figure 1. The anatomy of the human term placenta and amniotic membrane. (A) Schematic section of the human term placenta; (B) Schematic of amnion structure.
hADSCs are easily isolated and propagated ex vivo. hAMSCs show a fibroblast-like morphology in culture[24][25], while hAESCs exhibit a cobblestone-like morphology[23]. Compared with stem cells from other sources, hADSCs have the following advantages: (1) Easy to obtain, abundant sources, and no ethical and moral disputes: as the remaining after fetal birth the amniotic membrane can be used for separation of hAMSCs and hAESCs, which will not harm the donors; (2) No tumorigenicity: numerous studies showed that hADSCs had no proliferation and growth on soft agar in vitro, no colonies formed, no teratoma formation after implanting NOD-SCID mice in vivo[24]; (3) Low immunogenicity and high histocompatibility: hADSCs were considered as the immune-privileged cells and showed remarkable characteristics of low immunogenicity[26][27]. hADSCs had a low expression of the major histocompatibility class I antigen (HLA-ABC), no expressions of the major histocompatibility class II antigen (HLA-DR) and β2 microglobulin[28][29][30][31], importantly, the cells did not express HLA-ABC costimulatory molecules such as CD80, CD86 and CD40[32][33]. It has been reported that transplantation of hAMSCs into humans to treat lysosomal diseases showed no obvious rejection[34]. A recent study also demonstrated that intravenous administration of hAESCs did not result in haemolysis, allergic reactions, toxicity or tumor formation[35]. Akle et al. reported that an immunotype-mismatched human amniotic membrane did not elicit a host immune response when transplanted under the volunteers’ skin[36]. However, a few studies have highlighted that human amniotic cells might not actually be considered immune privileged, but, on the contrary, could stimulate both an innate and adaptive immune response, indicating that the possible co-immunostimulatory effects of amniotic stem cells[17][37].
2.2. The Molecular Markers of hADSCs
Both hAMSCs and hAESCs expressed the classical mesenchymal stem cells (MSCs) markers such as CD90, CD44, CD73, and CD105[8], and lack of cell surface markers such as CD45, CD34, CD45, HLA-CR, CD80, CD86. The molecular markers of hAMSCs and hAESCs are shown in Table 1. Expressions of MSCs markers in hAMSCs indicated that the cells possess the attractive clinical benefits of MSCs due to immune-privilege and the ability for immunomodulation.
Table 1. Expression of markers of human amniotic mesenchymal stem cells and amniotic epithelial stem cells.
| hADSCs | Epithelial Markers | Mesenchymal Stem Cell Markers | Pluripotent Markers | Hematopoietic Marker | The Major Histocompatibility Complex and Their Co-Stimulatory Molecules |
References | |
|---|---|---|---|---|---|---|---|
| Positive | Positive | Positive | Negative | Positive | Negative | ||
| hAESCs | Cytokeratin, E-cadherin, CD49f, CD326, |
CD29, CD166, | OCT4, NANOG, SSEA4, TRA-1-60 | CD34, CD45 | HLA-DR, HLA-DQ | Yang et al. [35] | |
| CK19 | CD29, CD44, CD73, CD90, CD105, | SSEA-4, SOX2, OCT-4 | CD31, CD34, CD45, CD49d | HLA-DR | Wu et al. [38] | ||
| CK7, E-cadherin | CD29, CD73, CD105 |
OCT4, NANOG, SSEA4 | CD34, CD45 | HLA-ABC | HLA-DR, CD80, CD86, CD40 | Liu et al. [23] | |
| E-cadherin | OCT4, SOX2, NANOG, TFE3, KLF4, SSEA3, SSEA4, TRA-1-60, REX1 |
Castro et al. [39] | |||||
| E-cadherin, CD49f, CK7, EpCAM | CD44, CD90, CD105, CD146, PDGFR-b, CD29 | CD45 | HLA-A-B-C | HLA-DP-DQ-DR, CD80, CD86, CD40 | Pratama et al. [33] | ||
| Cytokeratin | SSEA3, SSEA4, TRA-1-60, Oct-4 | CD34 | Evron et al. [40] | ||||
| CD29, CD73 | CD34, CD45 | HLA-ABC | HLA-A2, HLA-DQ, HLA-DR | Murphy et al. [30] | |||
| CD9, CD10, CD29, CD104, CD49f, CD105, CD44 | CD34, CD45 | HLA-ABC | HLA-DR, CD80, CD86, CD40 | Banas et al. [31] | |||
| SSEA-3, SSEA-4, TRA 1-60, TRA 1-81 | CD34 | Miki et al. [41] | |||||
| Cytokeratin, E-cadherin | CD29, CD166, CD90, | OCT4, NANOG, TRA 1-60, SOX2 | CD34, CD45 | HLA-DR, HLA-DQ | Yang et al. [35] | ||
| CK19, E-cadherin | CD29, CD44, CD90 | OCT4, SOX2, SSEA4, | CD34 | Wu et al. [42] | |||
| E-cadherin, | CD9, CD24, | SSEA-3, SSEA-4, TRA 1-60, TRA 1-81, Oct-4, Nanog |
CD34 | Miki et al. [36] | |||
| CD73, CD29, | Oct3/4, Sox2, Klf4, SSEA4, c-Myc. | CD34, CD45 | Koike et al. [43] | ||||
| hAMSCs | CD29, CD44, CD49d, CD73, CD90, CD105 |
SSEA-4, SOX2, OCT-4 | CD31, CD34, CD45 | HLA-DR | Wu et al. [38] | ||
| CD29, CD73, CD90, CD105 |
OCT4, NANOG, SSEA4 | CD34, CD45, CD133 | HLA-ABC | HLA-DR, CD80, CD86, CD40 | Liu and Li et al.[24][25] | ||
| CD44, CD90, CD105, CD146 | Oct-3/4 | CD45, CD34 | HLA-ABC | HLA-DR | Bačenková et al. [44] | ||
| CD90, CD44, CD73, CD166, CD105, CD29 | SSEA-4, STRO-1 | CD34, CD45 | Prado and Sugiura et al. [45][46] | ||||
| CD29, CD44, CD73, CD90, CD105 | SSEA-4, Oct4 | CD34, CD45, CD133 | HLA-ABC | HLA-DR | Coppi et al. [34] | ||
| CD105, CD117, | CD34 | HLA-ABC | HLA-DR | Borghesi et al. [47] | |||
| CD29, CD105 | Oct-3/4, SSEA-4, SOX2, NANOG, Rex-1 | HLA-A, HLA-DQB1 | Mihu et al. [48] | ||||
| CD44, CD90, CD105. CD90 | CD31, CD34 | Seo et al. [49] | |||||
| CD44, CD73, CD90, CD105, CD29, CD49f, CD271 | Oct3/4, Sox2, Klf4,SSEA4, Nanog, TRA1-60 | CD34, CD45 | Koike et al. [43] | ||||
| CD44, CD73, CD90, CD105, Vimentin |
OCT3/4, C-MYC, SOX2, NANOG, SSEA-3, SSEA-4 |
CD34, CD45 | HLA-DR | Nogami et al.[50] | |||
2.3. High Pluripotency of hADSCs
hADSCs have the potential to differentiate into all three germ layers when exposed to exogenous growth factors or chemicals[36]. Both hAMSCs and hAESCs expressed the typical surface markers of embryonic stem cells such as SSEA-3, SSEA-4, SOX-2, TRA1-60 and TRA1-81[51], especially the Oct-4 and Nanog[24], indicating the great potential of hAMSCs and hAESCs in regenerative medicine. So far, studies showed that hADSCs were able to differentiate into adipocytes[33][52], bone cells[34][38], nerve cells[39], cardiomyocytes[40][53], skeletal muscle cells [54][55], hepatocytes[34], hematopoietic cells[56], endothelial cells[57], kidney cells[58] and retinal cells[59][60](Figure 2).
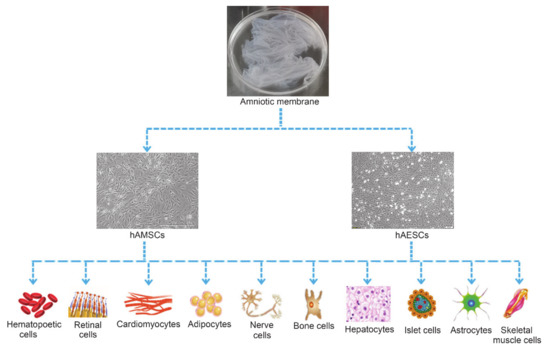
Figure 2. Multi-differentiation potential of hAMSCs and hAESCs.
Although various therapeutic approaches have been applied to promote angiogenesis, most of them were not able to fully mimic the process of natural vessel development. Amnion has both angiogenic and anti-angiogenic properties, which is surface dependent. The epithelial surface of amnion had inhibitory effects on vessel formation[50] and hAESCs were able to incorporate into the arterial wall without immunosuppression, but failed to improve vascular function[61]. However, the vessel length and sprout were increased in the amniotic mesenchymal side[12]. hAMSCs shared similar capability with bone marrow MSCs in neovascularization[62] and could initiate the cascade of signals by secreting factors needed for promoting formation of stable neo-vasculature and angiogenesis[62]. Several studies have evaluated the proangiogenic potential of hAMSCs which expressed high levels of representative proangiogenic genes VEGF-A, angiopoietin-1, HGF, and FGF-2 and anti-apoptotic factor AKT-1. By directly transplantation of hAMSCs into the ischemic hindlimbs of mice, the augmented blood perfusion and capillary density were observed, indicating that hAMSCs might promote the formation of neovascularization[63]. Moreover, hAMSCs exerted the beneficial paracrine effects on infarcted rat hearts through cardioprotection and angiogenesis[64].
hAMSCs and their conditional medium (hAMSC-CM) significantly improved the cutaneous blood flow after infusion into the ischemic leg whose femoral vessels were ligated[65]. König et al. observed that hAMSCs were able to take up acetylated low-density lipoprotein and form endothelial-like networks[66]. Studies showed that hAMSCs promoted angiogenesis by regulating the ERK1/2-MAPK signaling pathway[13] and that knockdown of lnRNA H19 significantly inhibited the angiogenic function of hAMSCs, in which the mechanism might be related to EZH2 degradation and VASH1 activation[67]. In addition, Tang et al. demonstrated that the angiogenesis of hAMSCs might be related to inhibit the functions of the Circ-ABCB10/miR-29b-3p/VEGFR, Circ-100290/miR-449a/eNOS, and Circ-100290/miR-449a/VEGFA axes in human umbilical vein endothelial cells[68][69]. Taken together, although the underlying mechanism of hAMSCs in angiogenesis is not fully elucidated the cells may become a useful reagent in various vascular diseases.
2.4. Immunosuppressive and Immunomodulatory Effects of hADSCs
hAMSCs-derived soluble factors including TGF-β, HGF, PGE2 and IDO suppress mitogen-induced peripheral blood mononuclear cell (PBMC) proliferation in a dose-dependent manner[70]. Wolbank et al. demonstrated the contact- and dose-dependent inhibitions of PBMC immune responses of hAMSCs and hAESCs[32]. Bulati et al. observed that INF-γ-induced immunomodulatory effects of hAMSC were dependent on the activated lymphomonocytes, cell-to-cell contact and soluble factors[71]. hAMSCs significantly inhibited the proliferation of stimulated lymphocytes and T cells [72]. Rossi et al. demonstrated that prostaglandins released by hAMSCs were responsible for the anti-proliferative effect on lymphocytes[70]. Meesuk et al. found hAMSCs exhibited immunosuppressive effect when they were co-cultured with activated T-cells by secreting indoleamine 2,3-dioxygenase[73]. Morandi et al. observed that HLA-G and -E molecules were involved in hAESCs-mediated suppression of T cell proliferation[74].
Furthermore, hADSCs also have immunomodulatory and immunosuppressive effects on inflammatory processes including reducing the activities of inflammatory cells and inhibiting migration of microglia and recruitment of immune cells to injury sites[16][17]. hADSCs also exhibited angiogenic, cytoprotective, anti-scarring, and antibacterial properties[75]. Therefore, it is reasonable to believe that hADSCs may be a potential cell source for cell-based therapy of diseases.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22020970
References
- De los Angeles, A.; Ferrari, F.; Xi, R.; Fujiwara, Y.; Benvenisty, N.; Deng, H.; Hochedlinger, K.; Jaenisch, R.; Lee, S.; Leitch, H.G.; et al. Hallmarks of pluripotency. Nature 2015, 525, 469–478.
- Yagi, M.; Yamanaka, S.; Yamada, Y. Epigenetic foundations of pluripotent stem cells that recapitulate in vivo pluripotency. Lab. Investig. 2017, 97, 1133–1141.
- Kang, N.H.; Hwang, K.A.; Kim, S.U.; Kim, Y.B.; Hyun, S.H.; Jeung, E.B.; Choi, K.C. Potential antitumor therapeutic strategies of human amniotic membrane and amniotic fluid-derived stem cells. Cancer Gene Ther. 2012, 19, 517–522.
- Han, C.-Y.; Liu, J.; Wan, F.; Tian, M.; Zhang, Y.-L.; He, Q.-H.; Si, Y.-C. Effects of Tianqijiangtang capsule on survival, self-renewal and differentiation of hippocampal neural stem cells of embryonic rats cultured in high glucose medium. Am. J. Transl. Res. 2019, 11, 5560–5572.
- Wang, C.; Spradling, A.C. An abundant quiescent stem cell population in Malpighian tubules protects principal cells from kidney stones. Elife 2020, 9, e54096.
- Loukogeorgakis, S.P.; de Coppi, P. Concise review: Amniotic fluid stem cells: The known, the unknown, and potential regenerative medicine applications. Stem Cells 2017, 35, 1663–1673.
- Vidane, A.S.; Souza, A.F.; Sampaio, R.V.; Bressan, F.F.; Pieri, N.C.; Martins, D.S.; Meirelles, F.V.; Miglino, M.A.; Ambrósio, C.E. Cat amniotic membrane multipotent cells are nontumorigenic and are safe for use in cell transplantation. Stem Cells Cloning 2014, 7, 71–78.
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Buhring, H.J.; Evangelista, M.; Hennerbichler, S.; Liu, B.; Magatti, M.; Mao, N.; et al. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311.
- Xu, H.; Zhang, J.; Tsang, K.S.; Yang, H.; Gao, W.-Q. Therapeutic potential of human amniotic epithelial cells on injuries and disorders in the central nervous system. Stem Cells Int. 2019, 2019, 5432301.
- Blum, B.; Benvenisty, N. The tumorigenicity of human embryonic stem cells. Adv. Cancer Res. 2008, 100, 133–158.
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676.
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260.
- Nauta, A.J.; Westerhuis, G.; Kruisselbrink, A.B.; Lurvink, E.G.A.; Willemze, R.; Fibbe, W.E. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 2006, 108, 2114–2120.
- Blokzijl, F.; de Ligt, J.; Jager, M.; Sasselli, V.; Roerink, S.; Sasaki, N.; Huch, M.; Boymans, S.; Kuijk, E.; Prins, P.; et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature 2016, 538, 260–264.
- Weeden, C.E.; Asselin-Labat, M.-L. Mechanisms of DNA damage repair in adult stem cells and implications for cancer formation. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 89–101.
- Abbasi-Kangevari, M.; Ghamari, S.-H.; Safaeinejad, F.; Bahrami, S.; Niknejad, H. Potential therapeutic features of human amniotic mesenchymal stem cells in multiple sclerosis: Immunomodulation, inflammation suppression, angiogenesis promotion, oxidative stress inhibition, neurogenesis induction, MMPs regulation, and remyelination stimulation. Front. Immunol. 2019, 10, 238.
- Magatti, M.; Vertua, E.; Cargnoni, A.; Silini, A.; Parolini, O. The immunomodulatory properties of amniotic cells: The two sides of the coin. Cell Transplant. 2018, 27, 31–44.
- Muttini, A.; Barboni, B.; Valbonetti, L.; Russo, V.; Maffulli, N. Amniotic epithelial stem cells: Salient features and possible therapeutic role. Sports Med. Arthrosc. Rev. 2018, 26, 70–74.
- Umezawa, A.; Hasegawa, A.; Inoue, M.; Tanuma-Takahashi, A.; Kajiwara, K.; Makino, H.; Chikazawa, E.; Okamoto, A. Amnion-derived cells as a reliable resource for next-generation regenerative medicine. Placenta 2019, 84, 50–56.
- Pogozhykh, O.; Prokopyuk, V.; Figueiredo, C.; Pogozhykh, D. Placenta and placental derivatives in regenerative therapies: Experimental studies, history, and prospects. Stem Cells Int. 2018, 2018, 4837930.
- Mathew, S.A.; Naik, C.; Cahill, P.A.; Bhonde, R.R. Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cell. Mol. Life Sci. 2020, 77, 253–265.
- Farhadihosseinabadi, B.; Farahani, M.; Tayebi, T.; Jafari, A.; Biniazan, F.; Modaresifar, K.; Moravvej, H.; Bahrami, S.; Redl, H.; Tayebi, L.; et al. Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 431–440.
- Liu, Q.W.; Liu, Q.Y.; Li, J.Y.; Wei, L.; Ren, K.K.; Zhang, X.C.; Ding, T.; Xiao, L.; Zhang, W.J.; Wu, H.Y.; et al. Therapeutic efficiency of human amniotic epithelial stem cell-derived functional hepatocyte-like cells in mice with acute hepatic failure. Stem Cell Res. Ther. 2018, 9, 321.
- Li, J.Y.; Ren, K.K.; Zhang, W.J.; Xiao, L.; Wu, H.Y.; Liu, Q.Y.; Ding, T.; Zhang, X.C.; Nie, W.J.; Ke, Y.; et al. Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem Cell Res. Ther. 2019, 10, 247.
- Liu, Q.-W.; Li, J.-Y.; Zhang, X.-C.; Liu, Y.; Liu, Q.-Y.; Xiao, L.; Zhang, W.-J.; Wu, H.-Y.; Deng, K.-Y.; Xin, H.-B. Human amniotic mesenchymal stem cells inhibit hepatocellular carcinoma in tumour-bearing mice. J. Cell. Mol. Med. 2020, 24, 10525–10541.
- Miki, T. A rational strategy for the use of amniotic epithelial stem cell therapy for liver diseases. Stem Cells Transl. Med. 2016, 5, 405–409.
- Insausti, C.L.; Blanquer, M.; Bleda, P.; Iniesta, P.; Majado, M.J.; Castellanos, G.; Moraleda, J.M. The amniotic membrane as a source of stem cells. Histol. Histopathol. 2010, 25, 91–98.
- Chiavegato, A.; Bollini, S.; Pozzobon, M.; Callegari, A.; Gasparotto, L.; Taiani, J.; Piccoli, M.; Lenzini, E.; Gerosa, G.; Vendramin, I.; et al. Human amniotic fluid-derived stem cells are rejected after transplantation in the myocardium of normal, ischemic, immuno-suppressed or immuno-deficient rat. J. Mol. Cell. Cardiol. 2007, 42, 746–759.
- Srinivasan, R.C.; Strom, S.C.; Gramignoli, R. Effects of cryogenic storage on human amnion epithelial cells. Cells 2020, 9, 1696.
- Murphy, S.; Rosli, S.; Acharya, R.; Mathias, L.; Lim, R.; Wallace, E.; Jenkin, G. Amnion epithelial cell isolation and characterization for clinical use. Curr. Protoc. Stem Cell Biol. 2010, 13, 1E.6.1–1E.6.25.
- Banas, R.A.; Trumpower, C.; Bentlejewski, C.; Marshall, V.; Sing, G.; Zeevi, A. Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Hum. Immunol. 2008, 69, 321–328.
- Wolbank, S.; Peterbauer, A.; Fahrner, M.; Hennerbichler, S.; van Griensven, M.; Stadler, G.; Redl, H.; Gabriel, C. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: A comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 2007, 13, 1173–1183.
- Pratama, G.; Vaghjiani, V.; Tee, J.Y.; Liu, Y.H.; Chan, J.; Tan, C.; Murthi, P.; Gargett, C.; Manuelpillai, U. Changes in culture expanded human amniotic epithelial cells: Implications for potential therapeutic applications. PLoS ONE 2011, 6, e26136.
- De Coppi, P.; Bartsch, G.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106.
- Yang, P.J.; Yuan, W.X.; Liu, J.; Li, J.Y.; Tan, B.; Qiu, C.; Zhu, X.L.; Qiu, C.; Lai, D.M.; Guo, L.H.; et al. Biological characterization of human amniotic epithelial cells in a serum-free system and their safety evaluation. Acta Pharmacol. Sin. 2018, 39, 1305–1306.
- Miki, T.; Strom, S.C. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006, 2, 133–142.
- Ding, C.; Li, H.; Wang, Y.; Wang, F.; Wu, H.; Chen, R.; Lv, J.; Wang, W.; Huang, B. Different therapeutic effects of cells derived from human amniotic membrane on premature ovarian aging depend on distinct cellular biological characteristics. Stem Cell Res. Ther. 2017, 8, 173.
- Wu, Q.; Fang, T.; Lang, H.; Chen, M.; Shi, P.; Pang, X.; Qi, G. Comparison of the proliferation, migration and angiogenic properties of human amniotic epithelial and mesenchymal stem cells and their effects on endothelial cells. Int. J. Mol. Med. 2017, 39, 918–926.
- Garcia-Castro, I.L.; Garcia-Lopez, G.; Avila-Gonzalez, D.; Flores-Herrera, H.; Molina-Hernandez, A.; Portillo, W.; Ramon-Gallegos, E.; Diaz, N.F. Markers of pluripotency in human amniotic epithelial cells and their differentiation to progenitor of cortical neurons. PLoS ONE 2015, 10, e0146082.
- Evron, A.; Goldman, S.; Shalev, E. Human amniotic epithelial cells cultured in substitute serum medium maintain their stem cell characteristics for up to four passages. Int. J. Stem Cells 2011, 4, 123–132.
- Miki, T.; Lehmann, T.; Cai, H.; Stolz, D.B.; Strom, S.C. Stem cell characteristics of amniotic epithelial cells. Stem Cells 2005, 23, 1549–1559.
- Wu, X.; Gao, F.; Wu, Y.; Sun, R.; Guan, W.; Tian, X. Isolation and biological characteristics of sheep amniotic epithelial cells. Cytotechnology 2019, 71, 539–551.
- Koike, C.; Zhou, K.; Takeda, Y.; Fathy, M.; Okabe, M.; Yoshida, T.; Nakamura, Y.; Kato, Y.; Nikaido, T. Characterization of amniotic stem cells. Cell. Reprogram. 2014, 16, 298–305.
- Bačenková, D.; Rosocha, J.; Tóthová, T.; Rosocha, L.; Šarisský, M. Isolation and basic characterization of human term amnion and chorion mesenchymal stromal cells. Cytotherapy 2011, 13, 1047–1056.
- Díaz-Prado, S.; Muiños-López, E.; Hermida-Gómez, T.; Rendal-Vázquez, M.E.; Fuentes-Boquete, I.; de Toro, F.J.; Blanco, F.J. Multilineage differentiation potential of cells isolated from the human amniotic membrane. J. Cell. Biochem. 2010, 111, 846–857.
- Sugiura, R.; Ohnishi, S.; Ohara, M.; Ishikawa, M.; Miyamoto, S.; Onishi, R.; Yamamoto, K.; Kawakubo, K.; Kuwatani, M.; Sakamoto, N. Effects of human amnion-derived mesenchymal stem cells and conditioned medium in rats with sclerosing cholangitis. Am. J. Transl. Res. 2018, 10, 2102–2114.
- Borghesi, J.; Ferreira Lima, M.; Mario, L.C.; da Anunciação, d.A.A.R.; Silveira Rabelo, A.C.; da Silva, G.K.C.M.; Assunpção Fernandes, F.; Miglino, M.A.; Oliveira Carreira, A.C.; Oliveira Favaron, P. Canine amniotic membrane mesenchymal stromal/stem cells: Isolation, characterization and differentiation. Tissue Cell 2019, 58, 99–106.
- Mihu, C.M.; Rus Ciucă, D.; Soritău, O.; Suşman, S.; Mihu, D. Isolation and characterization of mesenchymal stem cells from the amniotic membrane. Rom. J. Morphol. Embryol. 2009, 50, 73–77.
- Seo, M.-S.; Park, S.-B.; Kim, H.-S.; Kang, J.-G.; Chae, J.-S.; Kang, K.-S. Isolation and characterization of equine amniotic membrane-derived mesenchymal stem cells. J. Vet. Sci. 2013, 14, 151–159.
- Nogami, M.; Tsuno, H.; Koike, C.; Okabe, M.; Yoshida, T.; Seki, S.; Matsui, Y.; Kimura, T.; Nikaido, T. Isolation and characterization of human amniotic mesenchymal stem cells and their chondrogenic differentiation. Transplantation 2012, 93, 1221–1228.
- Díaz-Prado, S.; Muiños-López, E.; Hermida-Gómez, T.; Rendal-Vázquez, M.E.; Fuentes-Boquete, I.; de Toro, F.J.; Blanco, F.J. Isolation and characterization of mesenchymal stem cells from human amniotic membrane. Tissue Eng. Part C Methods 2011, 17, 49–59.
- Tsai, M.S.; Hwang, S.M.; Tsai, Y.L.; Cheng, F.C.; Lee, J.L.; Chang, Y.J. Clonal amniotic fluid-derived stem cells express characteristics of both mesenchymal and neural stem cells. Biol. Reprod. 2006, 74, 545–551.
- Bollini, S.; Pozzobon, M.; Nobles, M.; Riegler, J.; Dong, X.; Piccoli, M.; Chiavegato, A.; Price, A.N.; Ghionzoli, M.; Cheung, K.K.; et al. In vitro and in vivo cardiomyogenic differentiation of amniotic fluid stem cells. Stem Cell Rev. 2011, 7, 364–380.
- Pisciotta, A.; Riccio, M.; Carnevale, G.; Lu, A.; de Biasi, S.; Gibellini, L.; la Sala, G.B.; Bruzzesi, G.; Ferrari, A.; Huard, J.; et al. Stem cells isolated from human dental pulp and amniotic fluid improve skeletal muscle histopathology in mdx/SCID mice. Stem Cell Res. Ther. 2015, 6, 156.
- Zhang, D.; Yan, K.; Zhou, J.; Xu, T.; Xu, M.; Lin, J.; Bai, J.; Ge, G.; Hu, D.; Si, W.; et al. Myogenic differentiation of human amniotic mesenchymal cells and its tissue repair capacity on volumetric muscle loss. J. Tissue Eng. 2019, 10, 2041731419887100.
- Ditadi, A.; de Coppi, P.; Picone, O.; Gautreau, L.; Smati, R.; Six, E.; Bonhomme, D.; Ezine, S.; Frydman, R.; Cavazzana-Calvo, M.; et al. Human and murine amniotic fluid c-Kit+Lin− cells display hematopoietic activity. Blood 2009, 113, 3953–3960.
- Perin, L.; Sedrakyan, S.; da Sacco, S.; de Filippo, R. Characterization of human amniotic fluid stem cells and their pluripotential capability. Methods Cell Biol. 2008, 86, 85–99.
- Rota, C.; Imberti, B.; Pozzobon, M.; Piccoli, M.; de Coppi, P.; Atala, A.; Gagliardini, E.; Xinaris, C.; Benedetti, V.; Fabricio, A.S.; et al. Human amniotic fluid stem cell preconditioning improves their regenerative potential. Stem Cells Dev. 2012, 21, 1911–1923.
- Ghaderi, S.; Soheili, Z.S.; Ahmadieh, H.; Davari, M.; Jahromi, F.S.; Samie, S.; Rezaie-Kanavi, M.; Pakravesh, J.; Deezagi, A. Human amniotic fluid promotes retinal pigmented epithelial cells’ trans-differentiation into rod photoreceptors and retinal ganglion cells. Stem Cells Dev. 2011, 20, 1615–1625.
- Decembrini, S.; Cananzi, M.; Gualdoni, S.; Battersby, A.; Allen, N.; Pearson, R.A.; Ali, R.R.; de Coppi, P.; Sowden, J.C. Comparative analysis of the retinal potential of embryonic stem cells and amniotic fluid-derived stem cells. Stem Cells Dev. 2011, 20, 851–863.
- Vacz, G.; Cselenyak, A.; Cserep, Z.; Benko, R.; Kovacs, E.; Pankotai, E.; Lindenmair, A.; Wolbank, S.; Schwarz, C.M.; Horvathy, D.B.; et al. Effects of amniotic epithelial cell transplantation in endothelial injury. Interv. Med. Appl. Sci. 2016, 8, 164–171.
- Jiang, F.; Ma, J.; Liang, Y.; Niu, Y.; Chen, N.; Shen, M. Amniotic mesenchymal stem cells can enhance angiogenic capacity via MMPs in vitro and in vivo. BioMed Res. Int. 2015, 2015, 324014.
- Kim, S.-W.; Zhang, H.-Z.; Kim, C.E.; An, H.S.; Kim, J.-M.; Kim, M.H. Amniotic mesenchymal stem cells have robust angiogenic properties and are effective in treating hindlimb ischaemia. Cardiovasc. Res. 2012, 93, 525–534.
- Danieli, P.; Malpasso, G.; Ciuffreda, M.C.; Cervio, E.; Calvillo, L.; Copes, F.; Pisano, F.; Mura, M.; Kleijn, L.; de Boer, R.A.; et al. Conditioned medium from human amniotic mesenchymal stromal cells limits infarct size and enhances angiogenesis. Stem Cells Transl. Med. 2015, 4, 448–458.
- Kim, H.G.; Choi, O.H. Neovascularization in a mouse model via stem cells derived from human fetal amniotic membranes. Heart Vessel. 2011, 26, 196–205.
- König, J.; Huppertz, B.; Desoye, G.; Parolini, O.; Fröhlich, J.D.; Weiss, G.; Dohr, G.; Sedlmayr, P.; Lang, I. Amnion-derived mesenchymal stromal cells show angiogenic properties but resist differentiation into mature endothelial cells. Stem Cells Dev. 2012, 21, 1309–1320.
- Yuan, Z.; Bian, Y.; Ma, X.; Tang, Z.; Chen, N.; Shen, M. LncRNA H19 knockdown in human amniotic mesenchymal stem cells suppresses angiogenesis by associating with EZH2 and activating vasohibin-1. Stem Cells Dev. 2019, 28, 781–790.
- Tang, Z.; Tan, J.; Yuan, X.; Zhou, Q.; Yuan, Z.; Chen, N.; Shen, M. Circular RNA-ABCB10 promotes angiogenesis induced by conditioned medium from human amnion-derived mesenchymal stem cells via the microRNA-29b-3p/vascular endothelial growth factor A axis. Exp. Ther. Med. 2020, 20, 2021–2030.
- Tang, Z.; Wu, X.; Hu, L.; Xiao, Y.; Tan, J.; Zuo, S.; Shen, M.; Yuan, X. Circ-100290 positively regulates angiogenesis induced by conditioned medium of human amnion-derived mesenchymal stem cells through miR-449a/eNOS and miR-449a/VEGFA axes. Int. J. Biol. Sci. 2020, 16, 2131–2144.
- Rossi, D.; Pianta, S.; Magatti, M.; Sedlmayr, P.; Parolini, O. Characterization of the conditioned medium from amniotic membrane cells: Prostaglandins as key effectors of its immunomodulatory activity. PLoS ONE 2012, 7, e46956.
- Bulati, M.; Miceli, V.; Gallo, A.; Amico, G.; Carcione, C.; Pampalone, M.; Conaldi, P.G. The immunomodulatory properties of the human amnion-derived mesenchymal stromal/stem cells are induced by INF-γ produced by activated lymphomonocytes and are mediated by cell-to-cell contact and soluble factors. Front. Immunol. 2020, 11, 54.
- Dabrowski, F.A.; Burdzinska, A.; Kulesza, A.; Chlebus, M.; Kaleta, B.; Borysowski, J.; Zolocinska, A.; Paczek, L.; Wielgos, M. Mesenchymal stem cells from human amniotic membrane and umbilical cord can diminish immunological response in an in vitro allograft model. Gynecol. Obstet. Investig. 2017, 82, 267–275.
- Meesuk, L.; Tantrawatpan, C.; Kheolamai, P.; Manochantr, S. The immunosuppressive capacity of human mesenchymal stromal cells derived from amnion and bone marrow. Biochem. Biophys. Rep. 2016, 8, 34–40.
- Morandi, F.; Marimpietri, D.; Görgens, A.; Gallo, A.; Srinivasan, R.C.; El-Andaloussi, S.; Gramignoli, R. Human amnion epithelial cells impair T Cell proliferation: The role of HLA-G and HLA-E molecules. Cells 2020, 9, 2123.
- Carvajal, H.G.; Suárez-Meade, P.; Borlongan, C.V. Amnion-derived stem cell transplantation: A novel treatment for neurological disorders. Brain Circ. 2016, 2, 1–7.
