Curcuma longa L. rhizome essential oil is a valuable product in pharmaceutical industry due to its wide beneficial health effects.
- Curcuma longa
- essential oil
- extraction methods
- chemical composition
- agri-food industry
- antimicrobial
- herbicidal
- antioxidant
1. Introduction
Medicinal and aromatic plant species (MAPs) have been broadly exploited as food flavourings, medicinal agents, preservatives and ornaments, as well as beauty and personal delight products, becoming natural alternatives that offer reliability, safety and sustainability [1][2]. Amongst them, turmeric (Curcuma longa L., Zingiberaceae) is especially popular worldwide because of its attractive culinary, cosmetic and medicinal uses [3]. Specifically, the interest of this tuberous species resides in its exploitation as a colouring and flavouring agent, as well as in its numerous pharmacological activities, such as antioxidant, anticancer, anti-inflammatory, neuro- and dermoprotective, antiasthmatic or hypoglycaemic [4][5][6][7][8][9][10], being recently reported that turmeric can even potentially contribute against the life-threatening viral disease COVID-19 by inhibiting the main protease enzyme [11]. Most of these interesting features and properties principally come from the rhizome [3][12], a horizontal underground stem from which the shoots and roots arise [13]. It has distinctive organoleptic properties: a yellow/brown colour externally, with a deep orange inner part, a special aromatic smell and a bitter, hot taste. These characteristics make C. longa rhizome ideal for gastronomy. Especially, it is the principal ingredient of curry, for which it is probably popularly known [14][15][16].
Furthermore, rhizomes are a rich source of two major products with remarkable attributes: curcuminoids and essential oils [17]. On the one hand, curcuminoids are the responsible for the previously described orange-yellow colour [15]. They particularly refer to a group of three phenolic compounds, curcumin, desmethoxycurcumin and bisdesmethoxycurcumin, belonging to the diarylheptanoid family. They consist of a diketonic hydroxycarbon skeleton with different functional groups, depending on the curcuminoid [18][19]. Their content in the C. longa rhizome may vary according to many factors, such as the variety and geographic location, as well as cultivation and postharvest processing conditions [17][20]. For these secondary metabolites, turmeric is commonly employed as a spice and additives that provide colour and flavour in the food industry [21]. Additionally, they have demonstrated promising antioxidant and anti-inflammatory activities, being considered a valuable complementary therapy to pharmaceuticals in Crohn's, diabetes and cancer between other disorders [15][21][22]. Unfortunately, their poor solubility, low absorption and bioavailability, as well as high metabolic rate, limit their use for therapeutic purposes [23][24][25][26]. In fact, the major component curcumin has not been approved as a therapeutic agent yet due to its pharmacokinetics and physicochemical properties, despite it is generally considered a safe substance [24][27]. In response, curcuminoids have been associated with lipids, micelles, nanoparticles and other molecules to enhance their effects. An example is the binding of curcumin with phosphocaseins. This combination represents a suitable vector to deliver efficiently the compound, as well as other drugs and nutrients in general. New analogues with improved activity are being tried to develop from the original ones [21][28][29][30][31][32].
On the other hand, the essential oil is the one that provides the C. longa rhizome a particular spicy and aromatic flavour [3][15] with its distinctive chemical composition. In general, sesquiterpenes are the predominant phytochemical group in C. longa rhizome oil [33]. More concretely, ar-, α- and β–turmerones are usually the major and most representative components [34][35], although numerous intrinsic and extrinsic elements may influence in their quality and quantity [36][37][38][39][40][41]. Nevertheless, this chemical composition is different from the essential oil extracted from the aerial parts in which monoterpenes (α-phellandrene, terpinolene, 1,8-cineole, etc.) stand out [42][43][44][45][46]. Countless beneficial health effects have been attributed to C. longa rhizome oil as a consequence of this particular chemical composition: cardiovascular protection, antihyperlipidemic, antiglycaemic, antioxidant, antiplatelet, anti-inflammatory, antioxidant, antiarthritic, etc. [47]. Especially, abundant research has been focused on ar-turmerone, demonstrating its promising interesting medicinal properties, like the protection against the development of certain tumours [48][49], antifungal activity against dermatophytes [50], antiangiogenic effects [51], anticonvulsant properties [52] and treatment of neurodegenerative and other inflammatory diseases, such as psoriasis [53][54].
Nowadays, there is a growing demand of essential oils in the perfume and cosmetics, agriculture, pharmacy, food and beverage, as well as in many other, industries. One of the principal aims is to replace synthetic products with detrimental health and environmental effects [55]. In particular, numerous essential oils such as winter savory, peppermint, oregano, wintergreen and eucalypt, as well as many of their principal components (carvacrol, limonene, etc.) have already exhibited attractive and useful antimicrobial, herbicidal and antioxidant activities for the agri-food industry [56][57][58][59][60][61][62]. These data favour their potential use as natural preservatives to prevent the crop and food spoilage and extend the shelf-life, as well as weed control without significantly affecting the harvests.
2. Chemical Analysis of the Essential Oil Obtained from C. longa Rhizomes
The chemical composition of the essential oil obtained from C. longa rhizomes has been widely determined through Gas Chromatography-Mass Spectrometry (GC-MS) (Table 1), which is normally used for a sesquiterpenoid analysis [63] alone or combined with Gas Chromatography-Flame Ionisation detector (GC-FID) [64][65][66] to achieve a quantitative analysis. The determination of the chemical composition is key, because the components of the essential oil and their concentration can be considered a fingerprint conferring specific characteristics and properties [67].
As a general rule, oxygenated sesquiterpenes have been identified as the predominant ones (Table 1) and the principal reason of the biological activity of turmeric essential oil [68]. Concretely, turmerones (α-, β- and ar-) represent the major and the most distinctive individual components [69][70] (Table 1 and Figure 1). They give interesting properties to C. longa essential oil, such as anticancer, anti-inflammatory, antioxidant and the prevention of dementia [71][72][73][74][75]. Even they enhance the bioavailability and activity of other important turmeric components like curcumin [76][77][78]. In particular, ar-turmerone (6S-2-methyl-6-(4-methylphenyl) hept-2-en-4-one) has been identified as the leading one, followed by α- and β-, in C. longa rhizome oil (Table 1). Many authors have reported about the therapeutic potential of ar-turmerone and its numerous benefits for human health [75]. Lee demonstrated its antibacterial activity against human pathogens like Clostridium perfringens and Escherichia coli [79]. In the same year, he also reported a higher inhibitory effect than aspirin in platelet aggregation induced by collagen and arachidonic acid [80]. Other researchers have proposed ar-turmerone as a natural anticancer and cancer-preventive agent, being considered the α,β-unsaturated ketone of the molecule, the principal pharmacophore, for this activity [51][81][82][83]. ar-Turmerone has also been observed as useful in the prevention and attenuation of inflammatory diseases like psoriasis and neuronal ones [84][85].
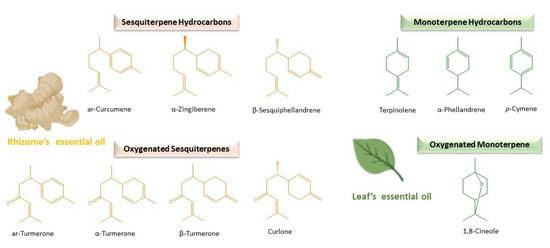
Figure 1. Main compounds found in the rhizomes and leaves of turmeric essential oils.
Oxygenated sesquiterpenes also constitute the predominant group in the essential oils obtained from the rhizome of other species included in the genus Curcuma [86]. For instance, curzerenone was the main compound in the rhizome oil of C. angustifolia and C. zedoaria; curdione was the major one in C. nankunshanensis, C. wenyujin and C. kwangsiensis; germacrone in C. sichuanensis and C. leucorhiza; β-elemenone in C. nankunshanensis var. nanlingensis; xanthorrhizol in C. xanthorrhiza and velleral in C. attenuata [86][87][88][89][90]. Turmerones are normally present, being considered the most representative components in general. Nevertheless, their amount may vary between species, probably due to the intrinsic differences between them [91]. The quantification of oxygenated sesquiterpenes, together with the identification of the secondary components, are key for the distinction and quality control of Curcuma spp. [17][92].
The sesquiterpenoids are generally followed by smaller quantities of sesquiterpene hydrocarbons in C. longa rhizome oil (Table 1 and Figure 1). This group is characterised by great structural diversity, providing a variety of fragrances and characteristic aromas to the essential oil [93]. Specifically, monocyclic bisabolane derivatives with a C6-ring formed in analogy to the menthane skeleton highlighted in turmeric essential oil obtained from rhizomes. Some examples are bisabolene isomers (β-bisabolene), α-zingiberene and ar-curcumene, characteristic of Curcuma spp. and ginger. β-Caryophyllene is also common, widely spread in food plants and derived from α-humulene, with a C9-ring fused to a cyclobutane ring [94]. Sesquiterpene hydrocarbons predominate over oxygenated ones in the rhizome oil of other Curcuma spp., such as C. aromatica (Sesquiterpene Hydrocarbons (SH): 8.30% ± 1.90% and Oxygenated Sesquiterpenes (OS): 7.10% ± 2.14%) and C. kwangsiensis var nanlingensis (SH: 9.76% ± 1.89% and OS: 6.80% ± 1.27%) [86].
The amount of monoterpene hydrocarbons and oxygenated monoterpenes are usually lower in most samples of rhizome essential oil of C. longa (Table 1). Contrarily, they constitute the most abundant group in the rhizome oil of other different Curcuma spp., such as C. amada [95], as well as in the essential oils obtained from the aerial parts of C. longa [17][96][97][98][99]. Regarding this, the yield of C. longa essential oil varied between the leaves (23%), rhizomes (48%) and rhizoids (27%), and the chemical composition was different between the leaf petiole, lamina and rhizoid oils (myrcene, p-cymene, etc.) compared to the stem and rhizome ones in which turmerones predominated [100]. α-Phellandrene, terpinolene and 1,8-cineole (Figure 1) are usually the most abundant compounds detected in the essential oil extracted from the leaves of C. longa [36][39][43][44], whereas turmerones are found in minor concentrations (Table 1) [70], being also usually found in the essential oils of the aerial parts of C. longa p-cymene, α-terpinene, myrcene and pinenes (Table 1) [96][97][99][101][102]. However, in samples of C. longa grown in Nigeria, the leaf essential oil was dominated by turmerones, like in rhizomes (Table 1) [103][104]. In addition, important concentrations of C8-aldehyde (20.58%) were found in the essential oil of C. longa leaves in a high-altitude research station in Odisha, India [102]. The concentration of these compounds can be increased by enhancing the leaf biomass production [105].
The aerial parts of C. longa normally end as waste products. An interest approach is their recycling to obtain biologically active compounds. In this sense, C. longa leaf essential oil and its principal component α-phellandrene have demonstrated remarkable insecticidal activity against Cochliomya macellaria, causative agents of myasis in humans and animals, as well as against Lucilia cuprina [106][107], being also a C. longa leaf essential oil highlight because of its medicinal and food-preservation properties, with a significant inhibition of microbial growth and toxin production [108][109].
On the other hand, several studies corroborate that the qualitative and quantitative chemical compositions of turmeric rhizomes essential oil may fluctuate according to many factors [86][110][111]. Sometimes, different chemical compositions come from the intrinsic characteristics of each genotype. In fact, certain traits of a specific variety of C. longa can influence the content of rhizome oil, representing good criteria for the selection of high-yield ones. Regarding this, an interesting study observed a direct relationship between plant height and rhizome oil content, as well as a negative correlation between the amount of essential oil in the dry leaf with the one contained in the fresh rhizome [112]. A clear example of genotype influence is the dissimilar chemical composition between yellow C. longa rhizome oil rich in oxygenated sesquiterpenes (ar-turmerone, turmerone, curlone, etc.) and red one with oxygenated monoterpenes (carvacrol, citral, methyl eugenol, geraniol, etc.) as principal compounds more similar to Origanum or Thymus spp. [113]. Indeed, the rhizome colour is closely related to the beneficial properties of C. longa [114]. The influence of the genotype or cultivars have also been reported by other authors who observed significant variations in the yield and chemical composition of rhizome oils of C. longa under similar climatic conditions [115][116][117].
Together with the genetic and environmental factors, the geographic location contributes to the different yields and quality of C. longa rhizome oils, even developing different chemotypes [39][70]. In India, the region of production determines the type of turmeric [118]. Samples from Nepal included α- and β-turmerones (8.19% and 17.74%, respectively) between other compounds like epi-α-patshutene (7.19%), β-sesquiphellandrene (4.99%), 1,4-dimethyl-2-isobutylbenzene (4.4%), (±)-dihydro-ar-turmerone (4.27%) and zingiberene (4.03%) [33]. The main components of the essential oil from Nigeria were ar-turmerone, α-turmerone and β-turmerone [103][119], while turmerones (approximately 37%), together with terpinolene (15.8%), zingiberene (11.8%) and β-sesquiphellandrene (8.8%), predominated in the rhizome oil from Reunion Island [97]. Turmerones still are also the predominant compounds in samples from Faisalabad (Pakistan) and Turkey [65][120]. In the South American continent, the essential oil isolated from rhizomes grown in Ecuador was rich in ar-turmerone (45.5%) and α-turmerone (13.4%), similar to Colombian samples, while that from Brazil was dominated by zingiberene (11%), sesquiphellandrene (10%), β-turmerone (10%) and α-curcumene (5%) [66][68][121].
The analysis of each C. longa habitat's conditions can help to predict the features of the resulting essential oil and enhance its yield and quality; what results especially important for its optimisation and commercialisation. Altitude, humidity, rainfall, temperature, soil pH, organic carbon, nitrogen, phosphorous and potassium are some of the factors that lead to wide variations in the yield and chemical composition of rhizome essential oil. From the development of predictive models and in vivo tests, the altitude, soil pH, nitrogen and organic carbon have been observed as enhancers of rhizome essential oil production. Amongst them, nitrogen and organic carbon raise the turmerone content concretely and phosphorous and potassium the oil yield [40][122][123][124]. Land configurations involving furrows and thatches surrounding C. longa reduce the loss of these soil nutrients, enhancing the rhizome yield [41].
The stage of maturity of C. longa rhizomes can also influence in the yield, chemical composition and properties of the essential oil. In relation to this, Garg et al. demonstrated that the percentage of the essential oil content widely varied between fresh and dried rhizomes of 27 accessions of C. longa in North India [125]. Similarly, Sharma et al. also observed certain variations in the qualitative and quantitative chemical compositions between the essential oils extracted from a mix of 5–10 month-old rhizomes and eight ones [101]. Furthermore, Singh et al. confirmed that fresh rhizome essential oil contained a major quantity of the active compound turmerone than dry ones, consequently having stronger activity [126]. A different trend was observed by Gounder et al., who reported the higher activity of cured (fresh rhizome boiled in water, dried in shade and polished) and dried rhizome oils over fresh ones [127], probably due to the lower percentage of ar-turmerone and β-turmerone. Anyway, the control of the drying conditions constitutes an important parameter in order to obtain the highest content of essential oil in the minimum time possible [128][129]. The sun and mechanical drying coexist as drying methods of C. longa rhizomes [118]. In particular, Monton et al. confirmed that one hour of microwave drying without conventional drying represented the optimum conditions to obtain the highest content of turmeric essential oil [129].
Table 1. Main components of C. longa essential oil according to the part of the plant used, origin, method of extraction and analysis. GC-MS: Gas Chromatography-Mass Spectrometry, GC-FID: Flame Ionisation Detector, SFE: Supercritical Fluid Extraction, SWE: Supercritical Water Extraction and: GC-FTIR: Gas Chromatography-Fourier-Transform Infrared.
| Part of Turmeric | Origin | Method of Extraction | Analysis | Yield | Main Components | Ref. |
|---|---|---|---|---|---|---|
|
Powdered rhizomes |
Nepal |
Hydrodistillation Clevenger |
GC-MS |
3.0% |
β–turmerone (17.74%), α-turmeron (8.19%), epi-α-patschutene (7.19%), β–sesquiphellandrene (4.99%), 1,4-dimethyl-2-isobutylbenzene (4.4%) |
[33] |
|
Pulverized rhizome |
India |
Steam distillation + vacuum distillation |
GC-MS |
1.6–46.6% |
Turmerones, l-zingiberene, β–sesquiphellandrene, ar-curcumene |
[71] |
|
Rhizomes |
Brazil |
Hydrodistillation assisted by microwave (HDAM) |
GC-MS |
0.6% |
ar-turmerone (50.37 ± 0.99%), β–turmerone (14.39 ± 0.33%), ar-curcumene (6.24 ± 0.21%) |
[130] |
|
Rhizomes |
Brazil |
HDAM + Cryogenic grinding (CG) |
GC-MS |
1.00% |
ar-turmerone (47.97 ± 1.19%), β–turmerone (13.70 ± 0.55%), ar-curcumene (5.94 ± 0.27%) |
[130] |
|
Rhizomes |
Brazil |
Steam distillation assisted by microwave (SDAM) |
GC-MS |
0.9% |
- |
[130] |
|
Rhizomes |
Brazil |
SDAM + CG |
GC-MS |
1.45% |
- |
[130] |
|
Powdered dried rhizome |
Serbia |
Hydrodistillation Clevenger |
GC-MS and GC-FID |
0.3 cm3/100 g |
ar-turmerone (22.7%), turmerone (26%) and curlone (16.8%) |
[65] |
|
Rhizomes |
Pakistan |
Hydrodistillation |
GC-MS |
0.673% |
ar-turmerone (25.3%), α-turmerone (18.3%) and curlone (12.5%) |
[120] |
|
Powdered rhizomes |
Thailand |
Hydrodistillation Clevenger |
GC-MS |
- |
ar-turmerone (43–49%), turmerone (13–16%) and curlone (17–18%) |
|
|
Dried rhizomes |
Brazil |
SFE |
GC-MS |
0.5–6.5 g/100 g |
ar-turmerone (20%) and ar-, α- and β–turmerones (~75%) |
[131] |
|
Dried rhizomes |
Brazil |
Extraction with volatile solvents |
GC-MS and CG-FID |
5.49% |
α-turmerone and β –turmerone (~8.7%), ar-turmerone (~3.6%) |
[65] |
|
Dried rhizomes |
Brazil |
Steam distillation |
GC-MS and CG-FID |
0.46% |
ar-turmerone (~12.8%), α-turmerone and β –turmerone (~4.1%) |
[65] |
|
Dried rhizomes |
China |
Steam distillation |
GC-MS |
4.50% w/w |
ar-turmerone (11.81%) |
[86] |
|
Dried rhizomes |
Nigeria |
Hydrodistillation Clevenger |
GC-MS |
1.33% w/w |
ar-turmerone (44.4%), α-turmerone (20.8%), β–turmerone (26.5%) |
[103] |
|
Dry rhizomes |
India |
Hydrodistillation Clevenger |
GC-MS |
2.9% |
ar-turmerone (21.4%), α-santalene (7.2%) and ar-curcumene (6.6%) |
[126] |
|
Dried rhizomes |
India |
Hydrodistillation Clevenger |
GC-MS |
3.05 ± 0.15% |
ar-turmerone (30.3%), α-turmerone (26.5%), β–turmerone (19.1%) |
[129] |
|
Cured rhizomes |
India |
Hydrodistillation Clevenger |
GC-MS |
4.45 ± 0.37% |
ar-turmerone (28.3%), α-turmerone (24.8%), β–turmerone (21.1%) |
[129] |
|
Dried root |
- |
SFE |
GC-MS |
2–5.3 wt% |
ar-turmerone (31–67.1%), β–turmerone (2–37.9%), α-turmerone (0–21.3%) |
[132] |
|
Fresh rhizomes |
Brazil |
Hydrodistillation Clevenger |
GC-MS |
1000 µL |
α-turmerone (42.6%), β –turmerone (16.0%) and ar-turmerone (12.9%) |
[34] |
|
Fresh rhizomes |
India |
Hydrodistillation Clevenger |
GC-MS |
0.6–2.1% |
Turmerone (35.24–44.22%) |
[39] |
|
Fresh rhizomes |
India |
Hydrodistillation Clevenger |
GC-MS |
0.8% |
α-turmerone (44.1%), β–turmerone (18.5%) and ar-turmerone (5.4%) |
[43] |
|
Fresh rhizomes |
India |
Hydrodistillation Clevenger |
GC-MS |
0.36% |
ar-turmerone (31.7%), α-turmerone (12.9%), β–turmerone (12.0%) and (Z)- β–ocimene (5.5%) |
[44] |
|
Fresh rhizomes |
India |
Modified distillation process |
GC-MS |
2.09–2.50% |
ar-turmerone (45.27%), curlone (5.6%), turmerone (4.4%), zingiberene (4.01%), ar-curcumene (4.01%), dehydrocurcumene (2.0%) |
[133] |
|
Fresh rhizome |
Malaysia |
SFE |
GC-MS |
- |
ar-turmerone (10.84–21.50%), turmerone (36.14–45.68%) and curlone (21.27–22.30%) |
[134] |
|
Fresh rhizomes |
Iran |
SWE |
GC-MS |
0.98% |
ar-turmerone (62.88%), curcumin (10.49%), β–sesquiphellandrene (9.62%), α-phellandrene (6.50%) |
[135] |
|
Fresh rhizomes |
Ecuador |
Steam distillation |
GC-FID and GC-MS |
0.8% v/w |
ar-turmerone (45.5%) and α-turmerone (13.4%) |
[66] |
|
Fresh rhizomes |
France |
Steam distillation |
GC-MS and GC-FTIR |
1.1% |
α-turmerone (21.4%), zingiberene (11.8%), terpinolene (15.8%), β–sesquiphellandrene (8.8%), ar-turmerone (7.7%) and β–turmerone (7.1%) |
[96] |
|
Fresh mature rhizomes |
Bhutan |
Hydrodistillation Clevenger |
GC-MS |
2–5.5% |
α-turmerone (30–32%), ar-turmerone (17–26%) and β–turmerone (15–18%) |
[101] |
|
Fresh rhizome |
India |
Steam distillation |
- |
2.03–6.50% |
- |
[118] |
|
Fresh rhizomes |
India |
Hydrodistillation Clevenger |
GC-MS |
1.8–3.73 mL/plant |
ar-turmerone (39.5–45.5%), curlone (9.8–11.7%), α-phellandrene (5.5–7.7%), eucalyptol (3.2–5.5%), β–himachalene (1.6–5.5%) and copen-11-ol (2.3–5.4%) |
[119] |
|
Fresh rhizomes |
Nigeria |
Hydrodistillation Clevenger |
GC-MS |
10.5 g (0.7% w/w) |
Turmerone (35.9%), α-phellandrene (15.5%), curlone (12.9%), 1,8-cineole (10.3%) and ar-turmerone (10.0%) |
[119] |
|
Fresh rhizomes |
Brazil |
Hydrodistillation Clevenger |
GC-MS |
0.70% |
Zingiberene (11%), sesquiphellandrene (10%), β–turmerone (10%) and α-curcumene (5%) |
[121] |
|
Mature fresh rhizomes |
India |
Hydrodistillation Clevenger |
GC-MS |
1.4% |
ar-turmerone (24.4%), α-turmerone (20.5%) and β–turmerone (11.1%) |
[128] |
|
Fresh rhizomes |
India |
Hydrodistillation Clevenger |
GC-MS |
3.52 ± 0.23% |
α-turmerone (33.5%), ar-turmerone (21.0%), β–turmerone (18.9%) |
[129] |
|
Semi dried leaves and fresh rhizomes |
India |
Continuous water circulation with steam distillation |
GC-MS |
Leaves: 2.75–2.83% Rhizomes: 2.38–2.48% |
Rhizome: Bisabolene (0.4%), ar-curcumene (2.3%), zingiberene (4.01%), dehydrocurcumene (2.0%), ar-turmerone (15.8%), turmerone (4.4%) and curlone (5.6%) |
[136] |
|
Semi-ripened and dried leaves |
India |
Water distillation techniques |
GC-MS |
0.25–0.28% v/w |
Terpinolene (33.0–57.6%), 1,8-cineole (1.9–7.9%), α-terpinene (1.7–3.9%), α-phellandrene (1.4–3.1%) |
[36] |
|
Partially senescenced leaves |
India |
Hydrodistillation Clevenger |
GC-MS |
- |
α-phellandrene, p-cymene, α-terpinolene, 1,8-cineole, p-cymen-8-ol |
[99] |
|
Dried leaves |
Nigeria |
Hydrodistillation Clevenger |
GC-MS |
0.67% w/w |
ar-turmerone (63.4%), α-turmerone (13.7%), β–turmerone (12.6%) |
[103] |
|
Dried leaves |
Nigeria |
Hydrodistillation Clevenger |
GC-MS |
0.67% w/w |
ar-turmerone (63.4%), α-turmerone (13.7%), β–turmerone (12.6%) |
[104] |
|
Leaves |
Bhutan |
Hydrodistillation Clevenger |
GC-MS |
0.3–0.42% |
α-phellandrene (18.2%), 1,8-cineole (14.6%) and p-cymene (13.3%) |
[101] |
|
Leaves |
India |
Hydrodistillation Clevenger |
GLC |
1.32% |
α-phellandrene (38.24%), C8-aldehyde (20.58%), 1,8-cineole (8.64%), α-pinene (2.88%) and β–pinene (2.36%) |
[102] |
|
Leaves |
Pakistan |
Hydrodistillation Reverse dean-stark method |
GC-MS |
145% |
Eucalyptol (10.27%), β–pinene (3.57%), 2-methylisoborneol (2.91%), limonene (2.73%), β–phellandrene (2.49%) |
[105] |
|
Fresh leaves |
India |
Hydrodistillation Clevenger |
GC-MS |
0.2–1.9% |
α-Phellandrene (30.82–39.85%), terpinolene (25.74–26.59%) and eucalyptol (7.52–7.66%) |
[39] |
|
Fresh leaves |
India |
Hydrodistillation Clevenger |
GC-MS |
0.65% |
α-phellandrene (53.4%), terpinolene (11.5%) and 1,8-cineole (10.5%) |
[43] |
|
Fresh leaves |
India |
Hydrodistillation Clevenger |
GC-MS |
0.53% |
α-phellandrene (9.1%), terpinolene (8.8%), 1,8-cineole (7.3%) and undecanol (7.1%) |
[44] |
|
Roughly crushed fresh leaves |
France |
Steam distillation |
GC-MS and GC-FTIR |
0.5% |
Terpinolene (77%), 1,8-cineole (4.6%), α-terpinene (3.7%), α-phellandrene (2.8%), myrcene (1.4%) and δ-3-carene (1.1%) |
[96] |
|
Fresh leaves |
India |
Steam distillation |
GC-MS |
0.15% |
Terpinolene (71.2%), 1,8-cineole (6.2%), p-cymen-9-ol (4.2%) |
[98] |
|
Fresh leaves and stems |
Colombia |
Steam distillation |
GC-MS |
- |
Turmerone (36.9%), α-turmerone (18.9%) and β–turmerone (13.6%) |
[68] |
|
Fresh aerial parts |
India |
SFE |
GC-MS |
2.8% |
p-cymene (25.4%), 1,8-cineole (18%), cis-sabinol (7.4%), β–pinene (6.3%) |
[97] |
|
Roughly crushed fresh flowers |
France |
Steam distillation |
GC-MS and GC-FTIR |
0.15% |
Terpinolene (67.4%), 1,8-cineole (4.6%), α-terpinene (4.4%), α-phellandrene (3.6%), myrcene (2%) and zingiberene (1.3%) |
[96] |
C. longa nutrition also has a significant impact in the yield and composition of rhizome oil. Especially, fertilizer use can enhance the productivity of volatile oil of C. longa rhizomes 6% [110]. Furthermore, a prior treatment with minerals during in vitro rhizome development followed by a fertilizer treatment in a greenhouse increases the percentage of volatiles in C. longa rhizomes. Particularly remarkable is the interaction of KNO3 and Ca2+, which favours the accumulation of sesquiterpenes in turmeric rhizome [137]. An interesting research proposed the use of arbuscular mycorrhizal fungi instead of chemical fertilizers in the cultivation of C. longa rhizomes. These optimise the absorption of nutrients and water, augment the metabolic activity of the plant, etc. In consequence, the root system becomes more robust, and the chemical composition of the essential oil is improved, increasing the production of certain compounds, including caryophyllene, α-curcumene, β-bisabolene and β-curcumene, using sustainable technologies [138][139]. Finally, the postharvest management of turmeric rhizomes also has a noteworthy influence on the quality of the derived products. Concretely, the boiling conditions, way of slicing, type of mill and speed of crushing and presence of heat and oxygen need to be controlled and standardised to obtain essential oils with certain characteristics [118].
In conclusion, the study of the chemical composition of the essential oil from the rhizome of C. longa gives us an idea of the characteristics and possible properties that it possesses. Sesquiterpenes are usually the main compounds in C. longa rhizome essential oil, highlighting the oxygenated turmerones followed by sesquiterpene hydrocarbons (Figure 1). However, the qualitative and quantitative chemical compositions of the essential oil can vary depending on the genetic and commented on factors. The knowledge of these can help to achieve a high-yield product with useful composition and properties for the agri-food industry.
3. Potential Applications of C. longa Essential Oil Obtained from Rhizomes in the Agri-Food Industry
Foodborne diseases, spoilage, insect and weed infestation are some common problems that cause significant economic losses to the agri-food industry. Chemical preservatives and pesticides have been widely exploited to maintain and enhance yields and productivity. However, the numerous handicaps derived from their overuse have been extensively described. As a result, sustainability has become an increasingly important subject in the agri-food industry. The characteristics of certain natural products, especially essential oils (zero waste), have become a matter of study as sustainable alternatives [140][141][142][143][144][145]. Amongst them, C. longa rhizome oil can take part in the safer and eco-friendly emergent agri-food industry due to its promising antimicrobial, herbicidal and antioxidant activities (Figure 2).
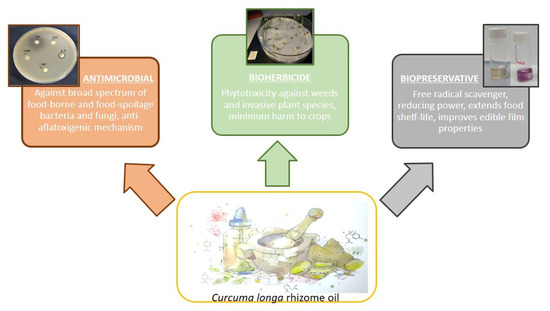
Figure 2. Representation of the roles that Curcuma longa rhizome oil can play in the safer and more sustainable emerging agri-food industry: antimicrobial, herbicidal and antioxidant activities.
This entry is adapted from the peer-reviewed paper 10.3390/plants10010044
References
- Singab, A.N.B.I. Medicinal & Aromatic Plants. Med. Aromat. Plants 2012, 1, 1000e109.
- Inoue, M.; Hayashi, S.; Craker, L.E. Role of medicinal and aromatic plants: Past, present, and future. In Pharmacognosy-Medicinal Plants; Perveen, S., Al-Taweel, A., Eds.; IntechOpen: London, UK, 2019; pp. 13–26, doi:10.5772/intechopen.82497.
- Saiz de Cos, P. Cúrcuma I (Curcuma longa L.). Reduca Ser. Botánica 2014, 7, 84–99.
- Araújo, C.A.C.; Leon, L.L. Biological activities of Curcuma longa L. Memórias Inst. Oswaldo Cruz 2001, 96, 723–728.
- Luthra, P.M.; Singh, R.; Chandra, R. Therapeutic uses of Curcuma longa (turmeric). Indian J. Clin. Biochem. 2001, 16, 153–160.
- Wickenberg, J.; Ingemansson, S.L.; Hlebowicz, J. Effects of Curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutr. J. 2010, 9, 43, doi:10.1186/1475-2891-9-43.
- Vaughn, A.R.; Branum, A.; Sivamani, R.K. Effects of turmeric (Curcuma longa) on skin health: A systematic review of the clinical evidence. Phytother. Res. 2016, 30, 1243–1264, doi:10.1002/ptr.5640.
- Dasgupta, A. Antiinflammatory herbal supplements. In Translational Inflammation; Actor, J.K., Smith, K.C., Eds.; Elsevier Inc.: London, UK, 2019; pp. 69–91, doi:10.1016/B978-0-12-813832-8.00004-2.
- El-Kenawy, A.E.-M.; Hassan, S.M.A.; Mohamed, A.M.M.; Mohammed, H.M.A.M. Tumeric or Curcuma longa Linn. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Sanches Silva, A., Eds.; Elsevier Inc.: London, UK, 2019; pp. 447–453, doi:10.1016/B978-0-12-812491-8.00059-X.
- Ortega, A.M.M.; Segura Campos, M.R. Medicinal plants and their bioactive metabolites in cancer prevention and treatment. In Bioactive Compounds: Health Benefits and Potential Applications; Campos, M.R.S., Ed.; Elsevier Inc.: Duxford, UK, 2019; pp. 83–109, doi:10.1016/B978-0-12-814774-0.00005-0.
- Rajagopal, K.; Varakumar, P.; Baliwada, A.; Byran, G. Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): An in silico approach. Future J. Pharm. Sci. 2020, 6, 104, doi:10.1186/s43094-020-00126-x.
- Alvis, A.; Arrazola, G.; Martínez, W. Evaluation of antioxidant activity and potential hydro-alcoholic extracts of curcuma (Curcuma longa). Inf. Tecnol. 2012, 23, 11–18, doi:10.4067/S0718-07642012000200003.
- Sawant, R.S.; Godghate, A.G. Qualitative phytochemical screening of rhizomes of Curcuma longa Linn. Int. J. Sci. Environ. Technol. 2013, 2, 634–641.
- Abraham, A.; Samuel, S.; Mathew, L. Pharmacognostic evaluation of Curcuma longa L. rhizome and standardization of its formulation by HPLC using curcumin as marker. Int. J. Pharmacogn. Phytochem. Res. 2018, 10, 38–42.
- Meng, F.; Zhou, Y.; Ren, D.; Wang, R. Turmeric: A review of its chemical composition, quality control, bioactivity, and pharmaceutical application. In Natural and Artificial Flavoring Agents and Food Dyes; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier Inc.: London, UK, 2018; pp. 299–350, doi:10.1016/B978-0-12-811518-3/00010-7.
- Carballido, E. Características de la Planta de la Cúrcuma. Available online: https://www.botanical-online.com/plantas-medicinales/curcuma-caracteristicas (accessed on 18 November 2020).
- Li, S.; Yuan, W.; Deng, G.; Wang, P.; Aggarwal, B.B. Chemical composition and product quality control of turmeric (Curcuma longa L.). Pharm. Crops 2011, 2, 28–54, doi:10.2174/2210290601102010028.
- González-Albadalejo, J.; Sanz, D.; Claramunt, R.M.; Lavandera, J.L.; Alkorta, I.; Elguero, J. Curcumin and curcuminoids: Chemistry, structural studies and biological properties. An. la Real Acad. Nac. Farm. 2015, 81, 278–310.
- Ramesh, T.N.; Paul, M.; Manikanta, K.; Girish, K.S. Structure and morphological studies of curcuminoids and curcuminoid mixture. J. Cryst. Growth 2020, 547, 125812, doi:10.1016/j.jcrysgro.2020.125812.
- Bambirra, M.L.A.; Junqueira, R.G.; Glória, M.B. Influence of post harvest processing conditions on yield and quality of ground turmeric (Curcuma longa L.). Braz. Arch. Biol. Technol. 2002, 45, 423–429.
- Amalraj, A.; Pius, A.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Chin. Med. Sci. 2017, 7, 205–233, doi:10.1016/j.jtcme.2016.05.005.
- Bengmark, S.; Mesa, M.D.; Gil, A. Plant-derived health: The effects of turmeric and curcuminoids. Nutr. Hosp. 2009, 24, 273–281.
- Tayyem, R.F.; Heath, D.D.; Al-delaimy, W.K.; Rock, C.L.; Tayyem, R.F.; Heath, D.D.; Al-delaimy, W.K.; Rock, C.L.; Tayyem, R.F.; Heath, D.D.; et al. Curcumin content of turmeric and curry powders. Nutr. Cancer 2006, 55, 126–131, doi:10.1207/s15327914nc5502_2.
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bioavailability. J. Herb. Med. 2015, 5, 57–70, doi:10.1016/j.hermed.2015.03.001.
- Akbar, A.; Kuanar, A.; Joshi, R.K.; Sandeep, I.S.; Mohanty, S. Development of prediction model and experimental validation in predicting the curcumin content of turmeric (Curcuma longa L.). Front. Plant Sci. 2016, 7, 1507, doi:10.3389/fpls.2016.01507.
- Tung, B.T.; Nham, D.T.; Hai, N.T.; Thu, D.K. Curcuma longa, the polyphenolic curcumin compound and pharmacological effects on liver. In Dietary Interventions in Liver Disease; Watson, R.R., Preedy, V.R., Eds.; Elsevier Inc.: London, UK, 2019; pp. 125–134, doi:10.1016/B978-0-12-814466-4.00010-0.
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother. Res. 2018, 32, 985–995, doi:10.1002/ptr.6054.
- Changtam, C.; De Koning, H.P.; Ibrahim, H.; Sajid, M.S.; Gould, M.K.; Suksamrarn, A. Curcuminoid analogs with potent activity against Trypanosoma and Leishmania species. Eur. J. Med. Chem. 2010, 45, 941–956, doi:10.1016/j.ejmech.2009.11.035.
- Priyadarsini, K.I. Chemical and structural features influencing the biological activity of curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100.
- Arshad, L.; Nasir, S.; Bukhari, A.; Jantan, I. An overview of structure-activity relationship studies of curcumin analogs as antioxidant and anti-inflammatory agents. Future Med. Chem. 2017, 9, 605–626, doi:10.4155/fmc-2016-0223.
- Noureddin, S.A.; El-shishtawy, R.M.; Al-footy, K.O. Curcumin analogues and their hybrid molecules as multifunctional drugs. Eur. J. Med. Chem. 2019, 182, 111631, doi:10.1016/j.ejmech.2019.111631.
- Benzaria, A.; Maresca, M.; Taieb, N.; Dumay, E. Interaction of curcumin with phosphocasein micelles processed or not by dynamic high-pressure. Food Chem. 2013, 138, 2327–2337, doi:10.1016/j.foodchem.2012.12.017.
- Devkota, L.; Rajbhandari, M. Composition of essential oils in turmeric rhizome. Nepal J. Sci. Technol. 2015, 16, 87–94, doi:10.3126/njst.v16i1.14361.
- Avanço, G.B.; Ferreira, F.D.; Bomfim, N.S.; Santos, P.A.D.S.R.D.; Peralta, R.M.; Brugnari, T.; Mallmann, C.A.; de Filho, B.A.A.; Mikcha, J.M.G.; Machinski, M., Jr. Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control 2017, 73, 806–813, doi:10.1016/j.foodcont.2016.09.032.
- Ferreira Guimares, A.; Andrade Vinhas, A.C.; Ferraz Gomes, A.; Souza, L.H.; Baier Krepsky, P. Essential oil of Curcuma longa L. rhizomes chemical composition, yield variation and stability. Quim. Nova 2020, 43, 909–913, doi:10.21577/0100-4042.20170547.
- Babu, G.D.K.; Shanmugam, V.; Ravindranath, S.D.; Joshi, V.P. Comparison of chemical composition and antifungal activity of Curcuma longa L. leaf oils produced by different water distillation techniques. Flavour Fragr. J. 2007, 22, 191–196, doi:10.1002/ffj.1780.
- Usman, L.A.; Hamid, A.A.; George, O.C.; Ameen, O.M.; Muhammad, N.O.; Zubair, M.F.; Lawal, A. Chemical composition of rhizome essential oil of Curcuma longa L. growing in North Central Nigeria. World J. Chem. 2009, 4, 178–181.
- Niranjan, A.; Singh, S.; Dhiman, M.; Tewari, S.K. Biochemical composition of Curcuma longa L. accessions. Anal. Lett. 2013, 46, 1069–1083, doi:10.1080/00032719.2012.751541.
- Akbar, A.; Kuanar, A.; Sandeep, I.S.; Kar, B.; Singh, S.; Mohanty, S.; Patnaik, J.; Nayak, S. GC-MS analysis of essential oil of some high drug yielding genotypes of turmeric (Curcuma longa L.). Int. J. Pharm. Pharm. Sci. 2015, 7, 35–40.
- Sandeep, I.S.; Das, S.; Nayak, S.; Mohanty, S. Chemometric profile of Curcuma longa L. towards standardization of factors for high essential oil yield and quality. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 949–957, doi:10.1007/s40011-016-0831-y.
- Choudhary, V.K.; Kumar, P.S. Weed suppression, nutrient leaching, water use and yield of turmeric (Curcuma longa L.) under different land configurations and mulches. J. Clean. Prod. 2019, 210, 795–803, doi:10.1016/j.jclepro.2018.11.071.
- Leela, N.K.; Tava, A.; Shafi, P.M.; Sinu, P.J.; Chempakam, B. Chemical composition of essential oils of turmeric. Acta Pharm. 2002, 52, 137–141.
- Raina, V.K.; Srivastava, S.K.; Syamsundar, K.V. Rhizome and leaf oil composition of Curcuma longa from the lower himalayan region of northern India. J. Essent. Oil Res. 2005, 17, 556–559, doi:10.1080/10412905.2005.9698993.
- Awasthi, P.; Dixit, S. Chemical composition of Curcuma longa leaves and rhizome oil from the plains of Northern India. J. Young Pharm. 2009, 1, 312–319, doi:10.4103/0975-1483.59319.
- Singh, S.; Panda, M.K.; Subudhi, E.; Nayak, S. Chemical composition of leaf and rhizome oil of an elite genotype Curcuma longa L. from South Eastern ghats of Orissa. J. Pharm. Res. 2010, 3, 1630–1633.
- Mishra, R.; Gupta, A.K.; Kumar, A.; Lal, R.K.; Saikia, D.; Chanotiya, C.S. Genetic diversity, essential oil composition, and in vitro antioxidant and antimicrobial activity of Curcuma longa L. germplasm collections. J. Appl. Res. Med. Aromat. Plants 2018, 10, 75–84, doi:10.1016/j.jarmap.2018.06.003.
- Dosoky, N.S.; Setzer, W.N. Chemical composition and biological activities of essential oils of Curcuma species. Nutrients 2018, 10, 10–17, doi:10.3390/nu10091196.
- Kim, D.; Suh, Y.; Lee, H.; Lee, Y. Immune activation and antitumor response of ar-turmerone on P388D1 lymphoblast cell implanted tumors. Int. J. Mol. Med. 2013, 31, 386–392, doi:10.3892/ijmm.2012.1196.
- Nair, A.; Amalraj, A.; Jacob, J.; Kunnumakkara, A.B.; Gopi, S. Non-curcuminoids from turmeric and their potential in cancer therapy and anticancer drug delivery formulations. Biomolecules 2019, 9, 13, doi:10.3390/biom9010013.
- Jankasem, M.; Wuthi-udomlert, M.; Gritsanapan, W. Antidermatophytic properties of ar-turmerone, turmeric oil, and Curcuma longa preparations. ISRN Dermatol. 2013, 2013, 250597, doi:10.1155/2013/250597.
- Yue, G.G.L.; Kwok, H.F.; Lee, J.K.M.; Jiang, L.; Chan, K.M.; Cheng, L.; Wong, E.C.W.; Leung, P.C.; Fung, K.P.; Lau, C.B.S. Novel anti-angiogenic effects of aromatic-turmerone, essential oil isolated from spice turmeric. J. Funct. Foods 2015, 15, 243–253, doi:10.1016/j.jff.2015.03.030.
- Orellana-paucar, A.M.; Afrikanova, T.; Thomas, J.; Aibuldinov, Y.K.; Dehaen, W.; De Witte, P.A.M.; Esguerra, C.V. Insights from zebrafish and mouse models on the activity and safety of ar-turmerone as a potential drug candidate for the treatment of epilepsy. PLoS ONE 2013, 8, e81634, doi:10.1371/journal.pone.0081634.
- Hucklenbroich, J.; Klein, R.; Neumaier, B.; Graf, R.; Fink, G.R.; Schroeter, M.; Rueger, M.A. Aromatic-turmerone induces neural stem cell proliferation in vitro and in vivo. Stem Cell Res. Ther. 2014, 5, 100.
- Li, Y.; Du, Z.; Li, P.; Yan, L.; Zhou, W.; Tang, Y. Aromatic-turmerone ameliorates imiquimod-induced psoriasis-like inflammation of BALB/c mice. Int. Immunopharmacol. 2018, 64, 319–325, doi:10.1016/j.intimp.2018.09.015.
- Khayreddine, B. Essential Oils, an Alternative to Synthetic Food Additives and Thermal Treatments; MedCrave Group LLC: Edmond, OK, USA, 2018; pp. 1–44.
- Boskovic, M.; Baltic, Z.M.; Ivanovic, J.; Duric, J.; Loncina, J.; Dokmanovic, M.; Markovic, R. Use of essential oils in order to prevent foodborne illnesses caused by pathogens in meat. Tehnol. Mesa 2013, 54, 14–20.
- Mihai, A.L.; Popa, M.E. Essential oils utilization in food industry—A literature review. Sci. Bull. Ser. F Biotechnol. 2013, 17, 187–192.
- Mihai, A.L.; Popa, M.E. Inhibitory effects of essential oils with potential to be used in food industry. Sci. Pharm. 2014, 18, 220–225.
- Bhavaniramya, S.; Vishnupriya, S.; Al-aboody, M.S. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55, doi:10.1016/j.gaost.2019.03.001.
- Boukhatem, M.N.; Boumaiza, A.; Nada, H.G.; Rajabi, M. Eucalyptus globulus essential oil as a natural food preservative: Antioxidant, antibacterial and antifungal properties in vitro and in a real food matrix (orangina fruit juice). Appl. Sci. 2020, 10, 5581, doi:10.3390/app10165581.
- Ibáñez, M.D.; Sanchez-Ballester, N.M.; Blázquez, M.A. Encapsulated limonene: A pleasant lemon-like aroma with promising application in the agri-food industry. A review. Molecules 2020, 25, 2598, doi:10.3390/molecules25112598.
- Ibáñez, M.D. Commercial Essential Oils: Sustainable Alternatives in the Agri-Food Industry. Ph.D. Thesis, Universitat de València, Valencia, Spain, 2019.
- Shang, Z.-P.; Xu, L.-L.; Lu, Y.-Y.; Guan, M.; Li, D.-Y.; Le, Z.-Y.; Bai, Z.-L.; Qiao, X.; Ye, M. Advances in chemical constituents and quality control of turmeric. World J. Tradit. Chin. Med. 2019, 5, 116–121, doi:10.4103/wjtcm.wjtcm_12_19.
- Manzan, A.C.C.M.; Toniolo, F.S.; Bredow, E.; Povh, N.P. Extraction of essential oil and pigments from Curcuma longa [L.] by steam distillation and extraction with volatile solvents. J. Agric. Food Chem. 2003, 51, 6802–6807, doi:10.1021/jf030161x.
- Stanojevic, J.; Stanojevic, L.; Cvetkovic, D.; Danilovic, B. Chemical composition, antioxidant and antimicrobial activity of the turmeric essential oil (Curcuma longa L.). Adv. Technol. 2015, 4, 19–25, doi:10.5937/savteh1502019s.
- Pino, J.A.; Fon-fay, F.M.; Falco, A.S.; Rodeiro, I. Chemical composition and biological activities of essential oil from turmeric (Curcuma longa L.) rhizomes grown in Amazonian Ecuador. Rev. CENIC 2018, 49, 1–8.
- Afzal, A.; Oriqat, G.; Khan, M.A.; Jose, J.; Afzal, M. Chemistry and biochemistry of terpenoids from Curcuma and related species. J. Biol. Act. Prod. Nat. 2013, 3, 1–55, doi:10.1080/22311866.2013.782757.
- Coy Barrera, C.C.A.; Eunice Acosta, G. Antibacterial activity and chemical composition of essential oils of rosemary (Rosemary officinalis), thyme (Thymus vulgaris) and turmeric (Curcuma longa) from Colombia. Rev. Cuba. Plantas Med. 2013, 18, 237–246.
- Jain, V.; Prasad, V.; Singh, S.; Pal, R. HPTLC method for the quantitative determination of ar-turmerone and turmerone in lipid soluble fraction from Curcuma longa. Nat. Prod. Commun. 2007, 2, 927–932, doi:10.1177/1934578x0700200912.
- Bahl, J.R.; Bansal, R.P.; Garg, S.N.; Gupta, M.M.; Singh, V.; Goel, R.; Kumar, S. Variation in yield of curcumin and yield and quality of leaf and rhizome essential oils among Indian land races of turmeric Curcuma longa L. Proc. Indian Natl. Sci. Acad. 2014, 80, 143–156, doi:10.16943/ptinsa/2014/v80i1/550.
- Matsumura, S.; Murata, K.; Zaima, N.; Yoshioka, Y.; Morimoto, M.; Kugo, H.; Yamamoto, A.; Moriyama, T.; Matsuda, H. Inhibitory activities of essential oil obtained from turmeric and its constituents against β-secretase. Nat. Prod. Commun. 2016, 11, 1785–1788, doi:10.1177/1934578x1601101203.
- Wegmann, M.; Lersch, P.; Wenk, H.H.; Klee, S.K.; Maczkiewitz, U.; Farwick, M.; Goldschmidt, E. Protective turmerones from Curcuma longa. Pers. Care 2009, 37–40.
- Bagad, A.S.; Joseph, J.A.; Bhaskaran, N.; Agarwal, A. Comparative evaluation of anti-inflammatory activity of curcuminoids, turmerones, and aqueous extract of Curcuma longa. Adv. Pharmacol. Sci. 2013, 2013, doi:10.1155/2013/805756.
- Del Prete, D.; Millán, E.; Pollastro, F.; Chianese, G.; Luciano, P.; Collado, J.A.; Munoz, E.; Appendino, G.; Taglialatela-Scafati, O. Turmeric sesquiterpenoids: Expeditious resolution, comparative bioactivity, and a new bicyclic turmeronoid. J. Nat. Prod. 2016, 79, 267–273, doi:10.1021/acs.jnatprod.5b00637.
- Prabhakaran Nair, K. Turmeric (Curcuma longa L.) and ginger (Zingiber o cinale Rosc.)—World’s Invaluable Medicinal Spices the Agronomy and Economy of Turmeric; Springer: Cham, Switzerland, 2019; pp. 1–568.
- Murakami, A.; Furukawa, I.; Miyamoto, S.; Tanaka, T.; Ohigashi, H. Curcumin combined with turmerones, essential oil components of turmeric, abolishes inflammation-associated mouse colon carcinogenesis. BioFactors 2013, 39, 221–232, doi:10.1002/biof.1054.
- Yue, G.G.L.; Cheng, S.W.; Yu, H.; Xu, Z.S.; Lee, J.K.M.; Hon, P.M.; Lee, M.Y.H.; Kennelly, E.J.; Deng, G.; Yeung, S.K.; et al. The role of turmerones on curcumin transportation and P-glycoprotein activities in intestinal caco-2 cells. J. Med. Food 2012, 15, 242–252, doi:10.1089/jmf.2011.1845.
- Yue, G.G.L.; Jiang, L.; Kwok, H.F.; Lee, J.K.M.; Chan, K.M.; Fung, K.P.; Leung, P.C.; Lau, C.B.S. Turmeric ethanolic extract possesses stronger inhibitory activities on colon tumour growth than curcumin—The importance of turmerones. J. Funct. Foods 2016, 22, 565–577, doi:10.1016/j.jff.2016.02.011.
- Lee, H.-S. Antimicrobial properties of turmeric (Curcuma longa L.) rhizome-derived ar-turmerone and curcumin. Food Sci. Biotechnol. 2006, 15, 559–563.
- Lee, H.S. Antiplatelet property of Curcuma longa L. rhizome-derived ar-turmerone. Bioresour. Technol. 2006, 97, 1372–1376, doi:10.1016/j.biortech.2005.07.006.
- Baik, K.U.; Jung, S.H.; Ahn, B.Z. Recognition of pharmacophore of ar-turmerone for its anticancer activity. Arch. Pharm. Res. 1993, 16, 254–256, doi:10.1007/BF02974492.
- Aratanechemuge, Y.; Komiya, T.; Moteki, H.; Katsuzaki, H.; Imai, K.; Hibasami, H. Selective induction of apoptosis by ar-turmerone isolated from turmeric (Curcuma longa L) in two human leukemia cell lines, but not in human stomach cancer cell line. Int. J. Mol. Med. 2002, 9, 481–484, doi:10.3892/ijmm.9.5.481.
- Cheng, S.B.; Wu, L.C.; Hsieh, Y.C.; Wu, C.H.; Chan, Y.J.; Chang, L.H.; Chang, C.M.J.; Hsu, S.L.; Teng, C.L.; Wu, C.C. Supercritical carbon dioxide extraction of aromatic turmerone from Curcuma longa Linn. Induces apoptosis through reactive oxygen species-triggered intrinsic and extrinsic pathways in human hepatocellular carcinoma HepG2 cells. J. Agric. Food Chem. 2012, 60, 9620–9630, doi:10.1021/jf301882b.
- Chen, M.; Chang, Y.Y.; Huang, S.; Xiao, L.H.; Zhou, W.; Zhang, L.Y.; Li, C.; Zhou, R.P.; Tang, J.; Lin, L.; et al. Aromatic-turmerone attenuates LPS-induced neuroinflammation and consequent memory impairment by targeting TLR4-dependent signaling pathway. Mol. Nutr. Food Res. 2018, 62, 1700281, doi:10.1002/mnfr.201700281.
- Yang, S.; Liu, J.; Jiao, J.; Jiao, L. Ar-turmerone exerts anti-proliferative and anti-inflammatory activities in HaCaT keratinocytes by inactivating hedgehog pathway. Inflammation 2020, 43, 478–486, doi:10.1007/s10753-019-01131-w.
- Zhang, L.; Yang, Z.; Wei, J.; Su, P.; Chen, D.; Pan, W.; Zhou, W.; Zhang, K.; Zheng, X.; Lin, L.; et al. Contrastive analysis of chemical composition of essential oil from twelve Curcuma species distributed in China. Ind. Crops Prod. 2017, 108, 17–25, doi:10.1016/j.indcrop.2017.06.005.
- Devi, L.R.; Rana, V.S.; Devi, S.I.; Blázquez, M.A.; Devi, L.R.; Rana, V.S.; Devi, S.I. Chemical composition and antimicrobial activity of the essential oil of Curcuma leucorhiza Roxb. J. Essent. Oil Res. 2012, 24, 533–538, doi:10.1080/10412905.2012.728089.
- Jantan, I.; Saputri, F.C.; Qaisar, M.N.; Buang, F. Correlation between chemical composition of Curcuma domestica and Curcuma xanthorrhiza and their antioxidant effect on human low-density lipoprotein oxidation. Evidence-Based Complement. Altern. Med. 2012, 2012, doi:10.1155/2012/438356.
- Singh, P.; Singh, S.; Kapoor, I.P.S.; Singh, G.; Isidorov, V.; Szczepaniak, L. Chemical composition and antioxidant activities of essential oil and oleoresins from Curcuma zedoaria rhizomes, part-74. Food Biosci. 2013, 3, 42–48, doi:10.1016/j.fbio.2013.06.002.
- Jena, S.; Ray, A.; Banerjee, A.; Sahoo, A.; Nasim, N.; Sahoo, S.; Kar, B.; Patnaik, J.; Panda, P.C.; Nayak, S. Chemical composition and antioxidant activity of essential oil from leaves and rhizomes of Curcuma angustifolia Roxb. Nat. Prod. Res. 2017, 31, 2188–2191, doi:10.1080/14786419.2017.1278600.
- Zhu, J.; Lower-Nedza, A.D.; Hong, M.; Jiec, S.; Wang, Z.; Yingmao, D.; Tschiggerl, C.; Bucar, F.; Brantner, A.H. Chemical composition and antimicrobial activity of three essential oils from Curcuma wenyujin. Nat. Prod. Commun. 2013, 8, 523–526, doi:10.1177/1934578x1300800430.
- Hong, S.L.; Lee, G.S.; Rahman, S.N.S.A.; Hamdi, O.A.A.; Awang, K.; Nugroho, N.A.; Malek, S.N.A. Essential oil content of the rhizome of Curcuma purpurascens Bl. (Temu Tis) and its antiproliferative effect on selected human carcinoma cell lines. Sci. World J. 2014, 2014, 397430, doi:10.1155/2014/397430.
- König, W.A.; Rieck, A.; Hardt, I.; Gehrcke, B.; Kubeczka, K.-H.; Muhle, H. Enantiomeric composition of the chiral constituents of essential oils. Part 2: Sesquiterpene hydrocarbons. J. High Resolut. Chromatogr. 1994, 17, 315–320, doi:10.1002/jhrc.1240170507.
- Chizzola, R. Regular monoterpenes and sesquiterpenes. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Heidelberg, Germany, 2013; pp. 2973–3008, doi:10.1007/978-3-642-22144-6_130.
- Tamta, A.; Prakash, O.; Punetha, H.; Pant, A.K. Chemical composition and in vitro antioxidant potential of essential oil and rhizome extracts of Curcuma amada Roxb. Cogent Chem. 2016, 2, 1–11, doi:10.1080/23312009.2016.1168067.
- Chane-Ming, J.; Vera, R.; Chalchat, J.C.; Cabassu, P. Chemical composition of essential oils from rhizomes, leaves and flowers of Curcuma longa L. from Reunion Island. J. Essent. Oil Res. 2002, 14, 249–251, doi:10.1080/10412905.2002.9699843.
- Garg, S.N.; Mengi, N.; Patra, N.K.; Charles, R.; Kumar, S. Chemical examination of the leaf essential oil of Curcuma longa L. from the north Indian plains. Flavour Fragr. J. 2002, 17, 103–104, doi:10.1002/ffj.1056.
- Pande, C.; Chanotiya, C.S. Constituents of the leaf oil of Curcuma longa L. from Uttaranchal. J. Essent. Oil Res. 2006, 18, 166–167, doi:10.1080/10412905.2006.9699056.
- Sharma, S.K.; Singh, S.; Tewari, S.K. Study of leaf oil composition from various accessions of Curcuma longa L. grown on partially reclaimed sodic soil. Int. J. Plant Environ. 2019, 5, 293–296, doi:10.18811/ijpen.v5i04.10.
- Bansal, R.P.; Bahl, J.R.; Garg, S.N.; Naqvi, A.A.; Kumary, S. Differential chemical compositions of the essential oils of the shoot organs, rhizomes and rhizoids in the turmeric Curcuma longa grown in indo-gangetic plains. Pharm. Biol. 2002, 40, 384–389, doi:10.1076/phbi.40.5.384.8458.
- Sharma, R.K.; Misra, B.P.; Sarma, T.C.; Bordoloi, A.K.; Pathak, M.G.; Leclercq, P.A. Essential oils of Curcuma longa L. from Bhutan. J. Essent. Oil Res. 1997, 9, 589–592, doi:10.1080/10412905.1997.9700783.
- Behura, S.; Sahoo, S.; Srivastava, V.K. Major constituents in leaf essential oils of Curcuma longa L. and Curcuma aromatica Salisb. Curr. Sci. 2002, 83, 1312–1313.
- Ajaiyeoba, E.O.; Sama, W.; Essien, E.E.; Olayemi, J.O.; Ekundayo, O.; Walker, T.M.; Setzer, W.N. Larvicidal activity of turmerone-rich essential oils of Curcuma longa leaf and rhizome from Nigeria on Anopheles gambiae. Pharm. Biol. 2008, 46, 279–282, doi:10.1080/13880200701741138.
- Essien, E.; Newby, J.; Walker, T.; Setzer, W.; Ekundayo, O. Chemotaxonomic characterization and in-vitro antimicrobial and cytotoxic activities of the leaf essential oil of Curcuma longa grown in Southern Nigeria. Medicines 2015, 2, 340–349, doi:10.3390/medicines2040340.
- Ananya, K.; Sujata, M.; Pande, M.K.; Sanghamitra, N. Essential oils from leaves of micropropagated turmeric. Curr. Sci. 2009, 96, 1166–1167.
- Chaaban, A.; Richardi, V.S.; Carrer, A.R.; Brum, J.S.; Cipriano, R.R.; Martins, C.E.N.; Navarro-Silva, M.A.; Deschamps, C.; Molento, M.B. Cuticular damage of Lucilia cuprina larvae exposed to Curcuma longa leaves essential oil and its major compound α-phellandrene. Data Br. 2018, 21, 1776–1778, doi:10.1016/j.dib.2018.11.001.
- Chaaban, A.; Gomes, E.N.; Richardi, V.S.; Martins, C.E.N.; Brum, J.S.; Navarro-Silva, M.A.; Deschamps, C.; Molento, M.B. Essential oil from Curcuma longa leaves: Can an overlooked by-product from turmeric industry be effective for myiasis control? Ind. Crops Prod. 2019, 132, 352–364, doi:10.1016/j.indcrop.2019.02.030.
- Sindhu, S.; Chempakam, B.; Leela, N.K.; Bhai, R.S. Chemoprevention by essential oil of turmeric leaves (Curcuma longa L.) on the growth of Aspergillus flavus and aflatoxin production. Food Chem. Toxicol. 2011, 49, 1188–1192, doi:10.1016/j.fct.2011.02.014.
- Parveen, Z.; Nawaz, S.; Siddique, S.; Shahzad, K. Composition and antimicrobial activity of the essential oil from leaves of Curcuma longa L. Kasur variety. Indian J. Pharm. Sci. 2013, 75, 117–122, doi:10.4103/0250-474X.113544.
- Kulpapangkorn, W.; Mai-Leang, S. Effect of plant nutrition on turmeric production. Procedia Eng. 2012, 32, 166–171, doi:10.1016/j.proeng.2012.01.1252.
- Dosoky, N.S.; Satyal, P.; Setzer, W.N. Variations in the volatile compositions of Curcuma species. Foods 2019, 8, 53, doi:10.3390/foods8020053.
- Yadav, R.; Lal, R.K.; Chanotiya, C.S.; Shanker, K.; Gupta, P.; Shukla, S. Prediction of genetic variability and character contribution using path analysis in turmeric (Curcuma longa L.) germplasm. Trends Phytochem. Res. 2019, 3, 91–100.
- Chowdhury, J.U.; Nandi, N.C.; Bhuiyan, M.N.I.; Mobarok, M.H. Essential oil constituents of the rhizomes of two types of Curcuma longa of Bangladesh. Bangladesh J. Sci. Ind. Res. 2008, 43, 259–266, doi:10.3329/bjsir.v43i2.970.
- Pal, K.; Chowdhury, S.; Dutta, S.K.; Chakraborty, S.; Chakraborty, M.; Pandit, G.K.; Dutta, S.; Paul, P.K.; Choudhury, A.; Majumder, B.; et al. Analysis of rhizome colour content, bioactive compound profiling and ex-situ conservation of turmeric genotypes (Curcuma longa L.) from sub-Himalayan terai region of India. Ind. Crops Prod. 2020, 150, 112401, doi:10.1016/j.indcrop.2020.112401.
- Kumar Rai, S.; Kumar Rai, K.; Pandey, N.; Kumari, A.; Tripathi, D.; Shashi Pandey, R. Varietal performance of turmeric (Curcuma longa L.) with special reference to curcumin and essential oil content under climatic conditions of Indogangetic plains. Veg. Sci. 2016, 43, 36–43.
- Shashidhar, M.D.; Hegde, N.K.; Hiremath, J.S.; Kukunoor, J.S.; Srikantprasad, D.; Patil, R.T. Evaluation of turmeric (Curcuma longa L.) genotypes for yield, curcumin and essential oil content in northern dry zone of Karnataka. J. Pharmacogn. Phytochem. 2018, SP3, 130–134.
- Sahoo, A.; Kar, B.; Jena, S.; Dash, B.; Ray, A.; Sahoo, S.; Nayak, S. Qualitative and quantitative evaluation of rhizome essential oil of eight different cultivars of Curcuma longa L. (Turmeric). J. Essent. Oil-Bear. Plants 2019, 22, 239–247, doi:10.1080/0972060X.2019.1599734.
- Plotto, A. Turmeric: Post-Production Management; Mazaud, F., Röttger, A., Steffel, K., Eds.; Rome, Italy, 2004; pp. 1–21.
- Oyemitan, I.A.; Elusiyan, C.A.; Onifade, A.O.; Akanmu, M.A.; Oyedeji, A.O.; McDonald, A.G. Neuropharmacological profile and chemical analysis of fresh rhizome essential oil of Curcuma longa (turmeric) cultivated in Southwest Nigeria. Toxicol. Rep. 2017, 4, 391–398, doi:10.1016/j.toxrep.2017.07.001.
- Naz, S.; Ilyas, S.; Parveen, Z.; Javed, S. Chemical analysis of essential oils from turmeric (Curcuma longa) rhizome through GC-MS. Asian J. Chem. 2010, 22, 3153–3158.
- Gonçalves, G.M.S.; Barros, P.P.; da Silva, G.H.; Fedes, G.R. The essential oil of Curcuma longa rhizomes as an antimicrobial and its composition by Gas Chromatography/Mass Spectrometry. Rev. Ciências Médicas 2019, 28, 1–10.
- Sandeep, I.; Sanghamitra, N.; Sujata, M. Differential effect of soil and environment onmetabolic expression of tumeric. Indian J. Exp. Biol. 2015, 53, 406–411.
- Sandeep, I.S.; Kuanar, A.; Akbar, A.; Kar, B.; Das, S.; Mishra, A.; Sial, P.; Naik, P.K.; Nayak, S.; Mohanty, S. Agroclimatic zone based metabolic profiling of turmeric (Curcuma longa L.) for phytochemical yield optimization. Ind. Crops Prod. 2016, 85, 229–240, doi:10.1016/j.indcrop.2016.03.007.
- Akbar, A.; Kuanar, A.; Patnaik, J.; Mishra, A.; Nayak, S. Application of artificial neural network modeling for optimization and prediction of essential oil yield in turmeric (Curcuma longa L.). Comput. Electron. Agric. 2018, 148, 160–178, doi:10.1016/j.compag.2018.03.002.
- Garg, S.N.; Bansal, R.P.; Gupta, M.M.; Kumar, S. Variation in the rhizome essential oil and curcumin contents and oil quality in the land races of turmeric Curcuma longa of North Indian plains. Flavour Fragr. J. 1999, 14, 315–318, doi:10.1002/(SICI)1099-1026(199909/10)14:53.0.CO;2-U.
- Singh, G.; Kapoor, I.P.S.; Singh, P.; de Heluani, C.S.; de Lampasona, M.P.; Catalan, C.A.N. Comparative study of chemical composition and antioxidant activity of fresh and dry rhizomes of turmeric (Curcuma longa Linn.). Food Chem. Toxicol. 2010, 48, 1026–1031, doi:10.1016/j.fct.2010.01.015.
- Gounder, D.K.; Lingamallu, J. Comparison of chemical composition and antioxidant potential of volatile oil from fresh, dried and cured turmeric (Curcuma longa) rhizomes. Ind. Crops Prod. 2012, 38, 124–131, doi:10.1016/j.indcrop.2012.01.014.
- Monton, C.; Luprasong, C.; Charoenchai, L. Convection combined microwave drying affect quality of volatile oil compositions and quantity of curcuminoids of turmeric raw material. Rev. Bras. Farm. 2019, 29, 434–440, doi:10.1016/j.bjp.2019.04.006.
- Monton, C.; Luprasong, C.; Charoenchai, L. Acceleration of turmeric drying using convection and microwave-assisted drying technique: An optimization approach. J. Food Process. Preserv. 2019, 43, e14096, doi:10.1111/jfpp.14096.
- Akloul, R.; Benkaci-Ali, F.; Eppe, G. Kinetic study of volatile oil of Curcuma longa L. rhizome and Carum carvi L. fruits extracted by microwave-assisted techniques using the cryogrinding. J. Essent. Oil Res. 2014, 26, 473–485, doi:10.1080/10412905.2014.942766.
- Carvalho, P.I.N.; Osorio-Tobón, J.F.; Rostagno, M.A.; Petenate, A.J.; Meireles, M.A.A. Techno-economic evaluation of the extraction of turmeric (Curcuma longa L.) oil and ar-turmerone using supercritical carbon dioxide. J. Supercrit. Fluids 2015, 105, 44–54, doi:10.1016/j.supflu.2015.03.020.
- Khanam, S. Influence of operating parameters on supercritical fluid extraction of essential oil from turmeric root. J. Clean. Prod. 2018, 188, 816–824, doi:10.1016/j.jclepro.2018.04.052.
- Sehgal, H.; Jain, T.; Malik, N.; Chandra, A.; Singh, S. Isolation and chemical analysis of turmeric oil from rhizomes. In Proceedings of the Chemical Engineering Towards Sustainable Development, 27–30December, 2016, Chennai, India, 2016; pp. 1–14.
- Haiyee, Z.A.; Shah, S.H.M.; Ismail, K.; Hashim, N.; Ismail, W.I.W. Quality parameters of Curcuma longa L. extracts by supercritical fluid extraction (SFE) and ultrasonic assisted extraction (UAE). Malays. J. Anal. Sci. 2016, 20, 626–632, doi:10.17576/mjas-2016-2003-23.
- Mottahedin, P.; Asl, A.H.; Khajenoori, M. Extraction of curcumin and essential oil from Curcuma longa L. by subcritical water via response surface methodology. J. Food Process. Preserv. 2017, 41, doi:10.1111/jfpp.13095.
- Chandra, A.; Prajapati, S.; Garg, S.K.; Rathore, A.K. Extraction of turmeric oil by continuous water circulation distillation. Int. J. Sci. Eng. Appl. Sci. 2016, 2, 2395–3470.
- El-Hawaz, R.F.; Grace, M.H.; Janbey, A.; Lila, M.A.; Adelberg, J.W. In vitro mineral nutrition of Curcuma longa L. affects production of volatile compounds in rhizomes after transfer to the greenhouse. BMC Plant Biol. 2018, 18, 122, doi:10.1186/s12870-018-1345-.
- de Ferrari, M.P.S.; dos Queiroz, M.S.; de Andrade, M.M.; Alberton, O.; Gonçalves, J.E.; Gazim, Z.C.; Magalhães, H.M. Substrate-associated mycorrhizal fungi promote changes in terpene composition, antioxidant activity, and enzymes in Curcuma longa L. acclimatized plants. Rhizosphere 2020, 13, 100191, doi:10.1016/j.rhisph.2020.100191.
- de Ferrari, M.P.S.; da Cruz, R.M.S.; dos Queiroz, M.S.; de Andrade, M.M.; Alberton, O.; Magalhães, H.M. Efficient ex vitro rooting, acclimatization, and cultivation of Curcuma longa L. from mycorrhizal fungi. J. Crops Sci. Biotechnol. 2020, 23, 469–482, doi:10.1007/s12892-020-00057-2.
- Blázquez, M.A. Role of natural essential oils in sustainable agriculture and food preservation. J. Sci. Res. Rep. 2014, 3, 1843–1860, doi:10.9734/JSRR/2014/1137.
- Walia, S.; Saha, S.; Tripathi, V.; Sharma, K.K. Phytochemical biopesticides: Some recent developments. Phytochem. Rev. 2017, 16, 989–1007, doi:10.1007/s11101-017-9512-6.
- Walia, S.; Saha, S.; Kundu, A. Environment friendly pesticides based on essential oils and their constituents. In Pesticides and Pests; Parmar, B.S., Singh, S.B., Walia, S., Eds.; Cambridge Scholars Publishin: Newcastle, UK, 2019; pp. 241–263.
- Garay, J. Review of essential oils: A viable pest control alternative. J. Hum. Ecol. 2020, 71, 13–22, doi:10.31901/24566608.2020/71.1-3.3244.
- Raveau, R.; Fontaine, J.; Sahraoui, A.L.-H. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, doi:10.3390/foods9030365.
- Werrie, P.-Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.-L. Phytotoxicity of essential oils: Opportunities and constraints for the development of biopesticides. A review. Foods 2020, 9, 1291, doi:10.3390/foods9091291.
