The immune system is a complex network dedicated to protecting an organism against harmful substances, including the eradication of invading pathogens or malignant cells, maintenance of specific memory lymphocytes and elimination of autoreactive immune cells to yield self-tolerances. Homeostasis of immune systems relies on two main components—the innate and adaptive immune responses, which are regulated by a series of cytokines that are released in response to certain stimulus. One of the most extensively studied cytokines is the common cytokine receptor common gamma chain (γc) family of cytokines, including interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15 and IL-21, which is named based on the usage of γc subunit for their receptors. This set of cytokines display broad pleiotropic actions to regulate both the innate and adaptive immune system, collectively contributing to the development of various immune cell populations, modulating cell differentiation, and either promoting the survival or inducing the apoptosis depending on the cellular context.
- cytokine
- interleukin-2
- interleukin-15
- immunotherapy
1. Introduction
IL-2 is the first member of this family to be discovered with a vital role in T cell development and expansion; IL-15 was later identified to share a number of biological activities with IL-2, which include stimulation of the proliferation and activation of T cells and NK cells, induction of B cell immunoglobulin synthesis and supporting cytolytic effector cell differentiation [1]. These redundancies could be explained by the common receptor subunits contained by receptors for IL-2 and IL-15, the shared β chain and γ chain, which trigger similar intracellular signaling pathways following binding with IL-2 or IL-15 [1]. Despite these similarities, IL-2 and IL-15 also display distinct functions in vivo, especially in adaptive immune responses. For example, IL-2 is required for the development and persistence of regulatory T (Treg) cells, and it is crucially involved in activation-induced cell death (AICD). By contrast, IL-15 is the major force for supporting natural killer (NK) cells and memory CD8+ T cell persistence, while its precise role with regard to Treg cells is still controversial [2]. It does not mediate AICD, but instead inhabits AICD induced by IL-2 [3]. In addition, recent studies have revealed the different ability of IL-2 and IL-15 to facilitate the activation and persistence of T and NK cells against various immune suppressive factors [4][5][6]. Their private receptor component, IL-2Rα or IL-15Rα, might contribute to these distinctive functions of IL-2 and IL-15, but further investigations are still required for better understanding of mechanism behind their differences.
Immunotherapy has improved the treatment outcomes in patients with cancer, and continuous efforts are devoted to exploiting the therapeutic potential of IL-2 and IL-15 based on their ability to expand and activate cytolytic lymphocytes in vivo. The utility of IL-2 as an antitumor agent was approved by the FDA in patients with advanced melanoma and renal cell carcinoma (RCC) decades ago [8]. Despite the encouraging clinical response rate, the accompanying severe side effects and toxicity of IL-2 therapy remains a major limitation. IL-15 has emerged as an alternative to IL-2 in cancer treatment, for its potent effects on cytolytic NK and T cells without inducing suppressive Treg cells. More recently, increasing insights on the biology of IL-2 and IL-15 have allowed remarkable translation advances in modulation of the pharmacokinetics of these cytokines to bypass limitations and boost efficacy. Meanwhile, there is a growing focus on using cytokines in combination strategies for synergistic immune enhancement.
2. The Biologic Profiles of IL-2 and IL-15
IL-2 and IL-15 are type I four α-helical bundle cytokines, referred to as the common γ receptor family of cytokines. This set of cytokines share the same receptor subunit γc and exhibit pleiotropic effects to modulate both innate and adaptive immune responses.
IL-2 was the first cytokine of this family to be identified; it was initially discovered from the supernatants of activated human T cells culture, which was a soluble factor that mediated T cell proliferation [8]. IL-2 is also the first cytokine approved by the FDA to be used in cancer treatment. While predominately secreted by CD4+ and CD8+ T cells following stimulation with antigen [9][10], a lesser amount of IL-2 is also produced by activated dendritic cells (DCs) [11], mast cells [12] and NKT cells [13]. Taking the advantage of Immgen data, Crellin et al. showed that ILC2 and ILC3 also produce il2 mRNA [14]. IL-2 conditionally deleted mice were recently generated, which can lead to better understanding of cellular sources of IL-2 within different tissues under certain conditions [15]. Transcription factors activated by the signals from T cell receptor (TCR) and other costimulations, including NF-κB [16], NFAT family protein [17], OCT-1 [18], FOS and JUN [19], directly triggered the activation of the Il2 gene [9][18]. As an autocrine/paracrine cytokine, the expression of IL-2 in T cells highly depends on the transcriptional regulation and mRNA stabilization, as well as the cellular activation state [20].
IL-15 first reported by two independent groups as a T cell proliferation factor, IL-15 exhibited its capability to mimic the IL-2-stimulated growth of T cells [21][22]. Through the signals emanated from their shared receptor subunit, IL-2/15 Rβ and γc, IL-15 also shares certain similar functions to IL-2, which include stimulation of the activated T cell proliferation, generation of cytotoxic effector T cells and the activation and persistence of NK cells [23]. They also facilitate the induction of immunoglobulin synthesis by B cells [24] and the regulation of lymphoid homeostasis [25]. However, unlike IL-2, IL-15 mRNA expression was detected in various tissues, both in hematopoietic and non-hematopoietic cells such as keratinocytes, nerve cells, stromal cells and fibroblasts [23][26]. Different from the widespread IL-15 mRNA expression, mature IL-15 protein production is mainly limited to DCs and monocytes/macrophages [1]. There are two isoforms of IL-15 mRNA with different signal peptides lengths, although those two isoforms yield same mature IL-15, they have distinct effect on the intracellular trafficking and secretion of IL-15 [27][29]. This indicates that IL-15 protein production is primarily controlled by the post-transcriptional stage, mainly the translation and intracellular trafficking process [29].
3. Receptors for IL-2 and IL-15
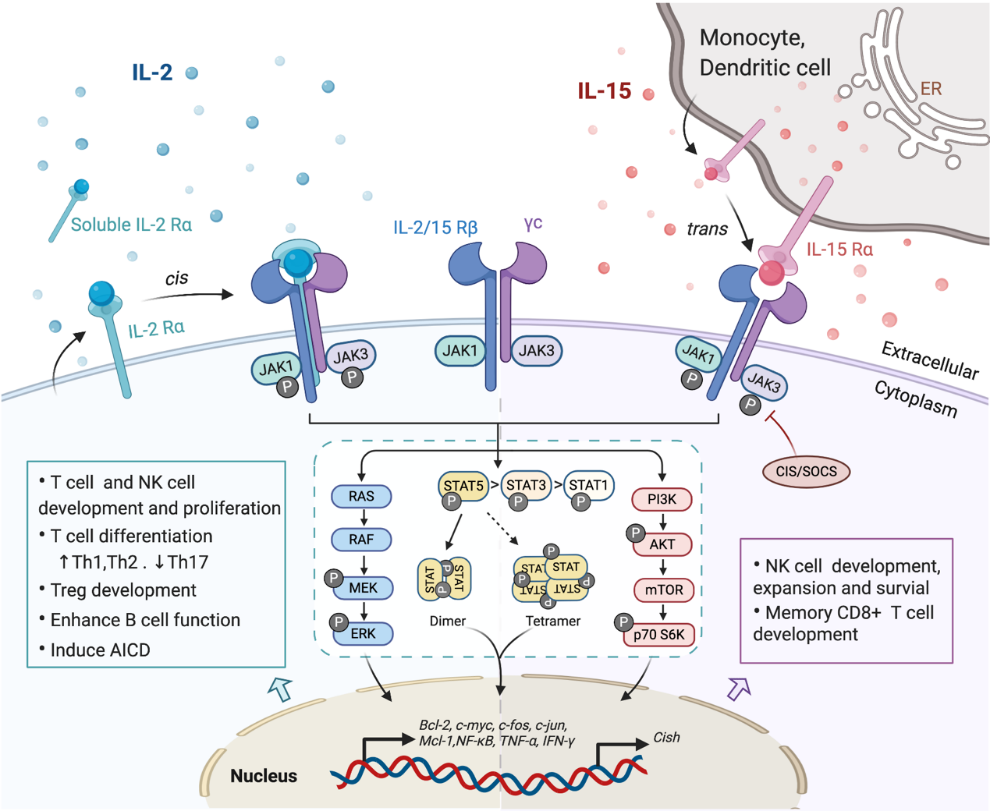
Investigating the components of receptor complexes and their downstream signals is essential to understand the biological effects of cytokines on the immune system. The receptors for IL-2 and IL-15 are both heterotrimeric, except for the common usage of cytokine receptor subunit, γc (also known as IL-2Rγ or CD132); they also share the beta subunit, referred to here as IL-2/15Rβ (also known as CD122) [30]. The third and unique receptor subunit for IL-2 and IL-15 is IL-2Rα and IL-15Rα, respectively (Figure 1). Most IL-2/15Rβ and γc are restrictively expressed by lymphohematopoietic cells, including T cells, NK cells, monocytes and neutrophils [9][31]. However, human fibroblasts were also reported to express functional IL-2/15Rβ and IL-2Rα, but the implications of this expression still remain unclear [32]. Ets1 family proteins, as well as Egr and Sp1, bind to IL-2/15Rβ promoter and trigger the expression in T cell and NK cells [33][34]. IL-2/15Rβ expression can be further regulated by IL-2, IL-4, PMA and TCR stimulation in both transcriptional and post-transcriptional phase [18]. The expression of γc has been shown to be boosted by IFNγ and IL-2 in monocytes whilst inhibited by TGF-β1 [35]. A post-transcriptional mechanism that mediates the soluble extracellular γc domain production and secretion in activated T cells has also been reported [36]. Although ubiquitination and cytokine-induced internalization evidently affect γc expression, little information is available concerning the cellular mechanisms controlling γc expression [37].
Figure 1. Interaction of interleukin-2 (IL-2) and IL-15 with their receptors and downstream signaling pathways. Receptors for IL-2 and IL-15 share two mutual submits, the common cytokine receptor γ-chain (γc) and the β-chain IL-2/15Rβ. Secreted IL-2 binds to its unique receptor submit IL-2Rα while membrane-associated IL-15 is trans-presented by monocytes or dendritic cells to NK cells or CD8+ T cells through binding with IL-15Rα, and then forms the high-affinity heterotrimeric receptor complex allowing the activation of downstream signaling. Both IL-2 and IL-15 mainly trigger the phosphorylation of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway, which could be limited by CIS/SOCS as a negative feedback. The phosphorylated STAT dimers or tetramers then translocate into the nucleus to regulate the transcription of target genes. Other pathways including RAS/Raf/MAPK and phosphoinositol 3-kinase (PI3K)/AKT are also activated by the ligation of these cytokines, contributing to the complex physical impacts of IL-2 and IL-15 on various immune cells.
The surface receptor complex for IL-2 signal consists of various combinations of the three IL-2R subunits. By stimulation via TCR or cytokines including IL-2, IL-7, IL-12, IL-15, the cytokine-specific IL-2Rα (CD25) is transcriptionally induced on T cells and NK cells, while is minimally detected on resting cells [18]. IL-2Rα is also found to be expressed by myeloid dendritic cells [38]. The isolated IL-2Rα binds IL-2 with low affinity (Kd~10−8 M) and serves only as a cytoplasmic anchor without transducing intracellular signals [9][39]. The combination of IL-2/15Rβ with γc forms intermediate-affinity heterodimeric receptor (Kd~10−9 M), while all three subsites together bind IL-2 with high affinity (Kd~10−11 M) and transduce intracellular signals [39][40]. In addition to the cell surface expression, IL-2Rα was also detected in certain diseases as in a soluble form (sIL-2R), including inflammatory disorders, transplantation rejection and most malignancy diseases [9][41][42]. The elevated level of sIL-2R ins serum is associated with disease progression and prognosis [9][42][43].
As the unique component of the IL-15 receptor complex, IL-15Rα is predominantly expressed on monocytes and dendritic cells, independent of IL-2Rβ and γc [1]. In contrast to IL-2Rα, IL-15Rα binds with IL-15 with high affinity by itself (Kd~10−11 M) and potentially mediates certain intracellular signals [44][45]. Unlike the other γc family cytokines which function as soluble ligands binding to and acting on the receptor expressing cell in cis, IL-15 primarily signals in trans as a cell-associated cytokine bounds to IL-15Rα expressing cells. The IL-15/IL-15Rα complex is then presented to IL-2/15Rβ and γc on bystander activated T cells or NK cells to form high-affinity immunological synapse, with signals induced similar to IL-2R [46][47]. Although IL-2 can be trans-presented by some IL-2Rα expressing DCs [48], it mainly signals in cis.
4. Common Downstream Pathways of IL-2 and IL-15 Receptors
In light of the shared receptor subunits (IL-2/15 Rβγ), IL-2 and IL-15 trigger several similar downstream signaling pathways including activation of common Janus kinase (JAKs)/signal transducer and activator of transcription (STATs), with JAK1 interacting with IL-2/15 Rβ and JAK3 with γc (Figure 1). STAT proteins, primarily STAT5A and STAT5B, are recruited to dock on IL-2/15 Rβγ, where they get phosphorylated, form dimers and translocate to the nucleus to bind with target genes [49][50]. Moreover, the N-terminal region of STAT5 can mediate oligomerization of dimers to allow the formation of tetramers and binding to tandem motifs, which is critical for IL-2-induced early cytokine responses and IL-15-induced NK cell maturation and survival [50][51]. Beyond the activation of JAK/STAT signaling pathway, IL-2/15R complex also mediate the stimulation of phosphoinositol 3-kinase (PI3K)/AKT pathway to promote cell survival and proliferation via subsequent mTOR activation [52]. Additionally, signaling through IL-2/IL-15R complex induces the expression of antiapoptotic protein Bcl-2 and activation of the RAS-Raf-MAPK pathway which regulate the transcription factor complexes containing FOS/JUN [53]. Among all those signaling pathways, phosphor-proteomic analysis of activated T cells suggested that 90% of IL-2-induced signaling is JAK kinase dependent [54]. It has also been shown that IL-15Rα could interact with TRAF2 to act on the transcription factor NF-κB [55], but further studies are still required to understand the intracellular trafficking of IL-15Rα. Apart from positive signals, IL-2 and IL-15 also induce the expression of negative regulators to prevent excessive responses. For example, the suppressors of cytokine signaling (SOCS) proteins (CIS, SOCS1) inhibit the JAK enzymatic activity via directly binding to JAK1, JAK2 and TYK2, to form a negative feedback loop limiting the cytokine signaling, thus SOCS protein has been considered as a novel checkpoint for NK cell immunotherapy [56][57].
5. Physiological Functions of IL-2 and IL-15
The common γ receptor family of cytokines collectively act to modulate development, proliferation, differentiation and survival of immune cells. Initially discovered as lymphocyte growth factor affecting the adaptive immune system, it has become clear that these cytokines are also involved in the innate immune response. Both IL-2 and IL-15 are critical regulators for innate lymphoid cells [58][59]. For example, IL-2 promotes elimination of pathogens by neutrophils, and IL-15 mediates the differentiation CD8αα+ intraepithelial lymphocytes via T-bet [60]. IL-2 and IL-15 mediate several similar functions on immune modulation as a consequence of sharing common receptor components and the JAK/STAT signaling pathway. These functions include the ability to promote proliferation and activation of CD4+ and CD8+ T cells, induce the differentiation of T helper cells and augment immunoglobin synthesis by activated B cells. Moreover, these two cytokines also play a crucial role in the generation and persistence of NK cells, potentiating the cytolytic activity of NK cells and CD8+T cells.
Despite these similarities, there are distinct differences between IL-2 and IL-15 with the actions on adaptive immune response (Figure 1). Apart from acting as a T cell growth factor, IL-2 also has a role in eliminating self-relative T cell via AICD [61], which is closely associated with the pathologic process of autoimmune diseases. In several systems, IL-15 has proven to be an antiapoptotic factor with potential to inhibit IL-2-induced AICD in vivo [3]. IL-2 usually favors the rapid proliferation of short-lived effector cells, while L-15 has its own unique effects on supporting the maintenance of long-lived memory phenotype CD8+ T cells and NK cells [62][63]. Although there have been a few reports showing that IL-15 is also involved in the development of Foxp3+ Treg cells [2][15][64][65], IL-2 is still recognized as the dominant driver for Treg cell development, homeostasis and fitness maintenance [66][67]. The capacity of IL-2 to activate both cytotoxic effector cells and Treg cells makes it a double-edged sword when utilized as an immunotherapeutic agent. To avoid this problem, different doses of IL-2 could be used based on the distinct IL-2R expression pattern on those two cell types. Compared with effector T cells, Treg cells express relatively higher levels of CD25, leading to a more frequent formation of high-affinity receptors for IL-2. As a result, while a high dose of IL-2 is required to preferentially expand effector T cells, Treg cells are able to respond rapidly to IL-2 at low concentrations (at single doses from 0.33 to 4.5 million IU) [68][69]. Additionally, the presence of IL-15 stimulates the production and recruitment of intestine intraepithelial γδ T cells; these results have not been observed with IL-2 [70][71]. Ex vivo cultured γδ T cells in the presence of IL-15 displayed prolonged survival and improved effector functions, as compared with IL-2. The production of αβ T cells from progenitor cells can be shifted to NK cells to a higher degree when encountering a high level of IL-15 [72]. Phenotypic differences such as cell size caused by IL-2 or IL-15 stimulation have less effect on signal transduction but more on the intensity and duration of the receptor signal [73].
Different transgenic mouse models have been used to confirm ex vivo functional observations of IL-2 and IL-15. IL-2 Rα deficient mice were more prone to develop autoimmune diseases, such as inflammatory bowel disease [74][75][76]. Massive enlargement of peripheral lymphoid organs associated with no selective T cell and B cell expansion was observed in IL-2 and IL-2 Rα deficient mice, due to the impairment of AICD and inhibited Treg cell development [77][78]. However, in mice with IL-15 or IL-15 Rα deficiency, no increased incidence of lymphoid enlargement or autoimmune disease was observed. Instead, they had remarkable reductions in NK cell, NKT cell and CD8+ memory T cell numbers in both periphery and thymus [79][80]. In the absence of NK cells and CD8+ memory T cells, IL-15-/- mice were more susceptible to various pathogens due to the compromised defense response. These selective lymphoid deficiencies could be reversed upon exogenous IL-15 provision, which further supports the critical biological role of this cytokine [80]. Trans-presentation of IL-15 mediated by IL-15Rα on antigen-presenting cells, such as DCs, is required for the generation and survival of NK cells, as well as for the longevity and avidity of antigen-specific CD8+T cells [46][47][63].
6. Immunomodulation of T and NK Cells in the Tumor Microenvironment
The development of tumors is often accompanied with an immunosuppressive microenvironment, hampering effector functions of cytotoxic lymphocytes, mostly CD8+ T cells and NK cells, to escape from immunosurveillance and promote progression. Overcoming the immunosuppression with sustained cytolytic activity of T and NK cells is required for efficient eradication of tumor cells. Recent studies have demonstrated different capacities of IL-2 and IL-15 in altering the susceptibility of T and NK cells to diverse immune suppressions. Discerning the roles of IL-2 and IL-15 in the regulation of antitumor immune responses is critical for the development of immunotherapeutic approaches against cancer.
CD8+T cells: Although IL-2 and IL-15 share many identical functions in the regulation of T cell as mentioned above, the distinct actions of these two cytokines on CD8+ T cells have been increasingly revealed. For instance, IL-15 is more efficient than IL-2 in cooperating with IL-21 to boost the expansion and effector function of splenic CD8+T cells, while for antigen-stimulated CD8+ T cells, IL-2 shows more potency in promoting protein synthesis than IL-15 [9][81]. The pivotal roles of IL-2 and IL-15 in activating CD8+T cells lead to the wide usage of these two cytokines in cancer immunotherapy. The in vivo persistence and activation of adoptively transferred T cells is usually maintained by IL-2 infusion, but with IL-15 as exogenous supplement or as transgene expressed, preclinical mouse studies demonstrated an enhanced antitumor capacity of CD8+T cells compared with IL-2 [82]. Recent studies have shown that IL-2 and IL-15 both triggered CD8+ T cell exhaustion by similarly inducing the expression of inhibitory receptor in vivo, particularly 2B4 and TIM-3, and selective abrogation of their common IL-2Rβchain could retain the inhibitory receptor induction [83]. In breast cancer, IL-15 provoked higher proliferation and IFNγ production of tumor-infiltrating CD8+ T cells than IL-2, and these strong but short-lived response could be diminished by the subsequently upregulated TIM-3 [84].
NK cells: The NK cell is a fundamental member of innate lymphocytes that mediates rapid and vigorous immunity against tumor cells. In adoptive NK cell therapy, both IL-2 and IL-15 can be used for the expansion and activation of NK cells in vitro before transfer. Although IL-2 is the most widely used in the clinic, IL-15 stimulation is reported to enable NK cells showing superior cytolytic performances and induce a memory-like NK cell population. In addition, IL-15 priming significantly ameliorated the antitumor response of CD56 bright NK cell subset [85]. Several studies have revealed the close association between IL-15 and the metabolic checkpoint kinase mTOR. Compared to IL-2, IL-15 augments stronger mTOR signaling, which is essential for the development and effector function of NK cells [86][87]. Meanwhile, continuous IL-15 exposure to NK cells could result in arrested cell cycle, diminished viability, reduced tumor cytolytic activity and metabolic deficiency, and this exhaustion status could be reversed by mTOR inhibitor [88]. TIM-3 could be induced on NK cells following the stimulation of IL-15 as well, which marks the maturation and cytotoxicity suppression status of NK cells [89].
In the context of supporting immune cell persistence in the immunosuppressive tumor microenvironment (TME), IL-2 and IL-15 have different potency in terms of regulating signaling pathway and protein synthesis. When encountered with abundant reactive oxidative species (ROS) in solid tumors, studies have shown that IL-15 stimulation upregulated the thioredoxin system in NK cells and T cells to confer increased tolerance towards oxidative stress [4][6]. As a complement, we found that IL-15 enhanced mTOR activity leading to higher levels of surface thiols on NK cells to neutralize extracellular ROS, compared with IL-2 [5]. Meanwhile, it has been reported that TGFβ could inhibit the activation and function of NK cells through curbing the IL-15-induced mTOR pathway [90]. These data indicate that IL-15 could be used as a promising antitumor agent to overcome immunosuppression in the TME.
7. Potential Mechanisms for the Distinction of IL-2 and IL-15
As the distinctive roles of IL-2 and IL-15 have been identified, it is vital to understand the potential mechanism underlying these contrasting functions. One factor is that these two cytokines are synthesized and secreted by different cells and tissues, which are regulated by distinct modes. Another reason could be the distinct ways these two cytokines interact with their receptors. As mentioned above, receptors for IL-2 and IL-15 comprise two subunits in common and mediate similar pathways including JAK/STAT, but they both have their private components, IL-2Rα and IL-15Rα, respectively. This means that the diverse physiological distribution of these two α-chains could also contribute to the biological differences of IL-2 and IL-15 in vivo. Moreover, a recent study has suggested that exposure to IL-15 causes the reduction in expression of IL-15Rα [47]. Apart from the similar signaling pathways, there are still several distinct downstream pathways through the receptor that have been detected. For example, T cell proliferation induced by IL-15 largely depends on FKBP12-mediated activation of p70S6 kinase, but FKBP12 is not indispensable for IL-2-induced proliferation [91]. Instead, the response of T cells to IL-2 requires another protein FKBP12.6, which is not involved in the response to IL-15 [1][91]. Additionally, as mentioned above, IL-15 triggers elevated mTOR signaling in NK cells compared with IL-2. To date, the molecular basis underlying the differences between IL-2 and IL-15 intracellular signaling has been poorly described; these preliminary findings require further investigations for optimizing clinical implications of IL-2 and IL-15.
This entry is adapted from the peer-reviewed paper 10.3390/cancers12123586
References
- Waldmann, T.A. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006, 6, 595–601, doi:10.1038/nri1901.
- Read, K.A.; Powell, M.D.; McDonald, P.W.; Oestreich, K.J. IL-2, IL-7, and IL-15: Multistage regulators of CD4(+) T helper cell differentiation. Exp. Hematol. 2016, 44, 799–808, doi:10.1016/j.exphem.2016.06.003.
- Marks-Konczalik, J.; Dubois, S.; Losi, J.M.; Sabzevari, H.; Yamada, N.; Feigenbaum, L.; Waldmann, T.A.; Tagaya, Y. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc. Natl. Acad. Sci. USA 2000, 97, 11445–11450, doi:10.1073/pnas.200363097.
- Mimura, K.; Kua, L.F.; Shimasaki, N.; Shiraishi, K.; Nakajima, S.; Siang, L.K.; Shabbir, A.; So, J.; Yong, W.P.; Kono, K. Upregulation of thioredoxin-1 in activated human NK cells confers increased tolerance to oxidative stress. Cancer Immunol. Immunother. CII 2017, 66, 605–613, doi:10.1007/s00262-017-1969-z.
- Yang, Y.; Neo, S.Y.; Chen, Z.; Cui, W.; Chen, Y.; Guo, M.; Wang, Y.; Xu, H.; Kurzay, A.; Alici, E.; et al. Thioredoxin activity confers resistance against oxidative stress in tumor-infiltrating NK cells. J. Clin. Investig. 2020, 130, 5508–5522, doi:10.1172/JCI137585.
- Kaur, N.; Naga, O.S.; Norell, H.; Al-Khami, A.A.; Scheffel, M.J.; Chakraborty, N.G.; Voelkel-Johnson, C.; Mukherji, B.; Mehrotra, S. T cells expanded in presence of IL-15 exhibit increased antioxidant capacity and innate effector molecules. Cytokine 2011, 55, 307–317, doi:10.1016/j.cyto.2011.04.014.
- Rosenberg, S.A. IL-2: The First Effective Immunotherapy for Human Cancer. J. Immunol. 2014, 192, 5451–5458, doi:10.4049/jimmunol.1490019.
- Morgan, D.A.; Ruscetti, F.W.; Gallo, R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 1976, 193, 1007–1008, doi:10.1126/science.181845.
- Liao, W.; Lin, J.X.; Leonard, W.J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013, 38, 13–25, doi:10.1016/j.immuni.2013.01.004.
- Paliard, X.; de Waal Malefijt, R.; Yssel, H.; Blanchard, D.; Chrétien, I.; Abrams, J.; de Vries, J.; Spits, H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J. Immunol. 1988, 141, 849–855.
- Granucci, F.; Vizzardelli, C.; Pavelka, N.; Feau, S.; Persico, M.; Virzi, E.; Rescigno, M.; Moro, G.; Ricciardi-Castagnoli, P. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat. Immunol. 2001, 2, 882–888, doi:10.1038/ni0901-882.
- Hershko, A.Y.; Suzuki, R.; Charles, N.; Alvarez-Errico, D.; Sargent, J.L.; Laurence, A.; Rivera, J. Mast cell interleukin-2 production contributes to suppression of chronic allergic dermatitis. Immunity 2011, 35, 562–571, doi:10.1016/j.immuni.2011.07.013.
- Yui, M.A.; Sharp, L.L.; Havran, W.L.; Rothenberg, E.V. Preferential activation of an IL-2 regulatory sequence transgene in TCR gamma delta and NKT cells: Subset-specific differences in IL-2 regulation. J. Immunol. 2004, 172, 4691–4699, doi:10.4049/jimmunol.172.8.4691.
- Crellin, N.K.; Trifari, S.; Kaplan, C.D.; Satoh-Takayama, N.; Di Santo, J.P.; Spits, H. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity 2010, 33, 752–764, doi:10.1016/j.immuni.2010.10.012.
- Owen, D.L.; Mahmud, S.A.; Vang, K.B.; Kelly, R.M.; Blazar, B.R.; Smith, K.A.; Farrar, M.A. Identification of Cellular Sources of IL-2 Needed for Regulatory T Cell Development and Homeostasis. J. Immunol. 2018, 200, 3926–3933, doi:10.4049/jimmunol.1800097.
- Gringhuis, S.I.; de Leij, L.F.; Verschuren, E.W.; Borger, P.; Vellenga, E. Interleukin-7 upregulates the interleukin-2-gene expression in activated human T lymphocytes at the transcriptional level by enhancing the DNA binding activities of both nuclear factor of activated T cells and activator protein-1. Blood 1997, 90, 2690–2700.
- Müller, M.R.; Rao, A. NFAT, immunity and cancer: A transcription factor comes of age. Nat. Rev. Immunol. 2010, 10, 645–656, doi:10.1038/nri2818.
- Kim, H.P.; Imbert, J.; Leonard, W.J. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 2006, 17, 349–366, doi:10.1016/j.cytogfr.2006.07.003.
- Mondino, A.; Whaley, C.D.; DeSilva, D.R.; Li, W.; Jenkins, M.K.; Mueller, D.L. Defective transcription of the IL-2 gene is associated with impaired expression of c-Fos, FosB, and JunB in anergic T helper 1 cells. J. Immunol. 1996, 157, 2048–2057.
- Lindstein, T.; June, C.H.; Ledbetter, J.A.; Stella, G.; Thompson, C.B. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science 1989, 244, 339–343, doi:10.1126/science.2540528.
- Grabstein, K.H.; Eisenman, J.; Shanebeck, K.; Rauch, C.; Srinivasan, S.; Fung, V.; Beers, C.; Richardson, J.; Schoenborn, M.A.; Ahdieh, M.; et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science 1994, 264, 965–968, doi:10.1126/science.8178155.
- Bamford, R.N.; Grant, A.J.; Burton, J.D.; Peters, C.; Kurys, G.; Goldman, C.K.; Brennan, J.; Roessler, E.; Waldmann, T.A. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc. Natl. Acad. Sci. USA 1994, 91, 4940–4944, doi:10.1073/pnas.91.11.4940.
- Waldmann, T.A.; Tagaya, Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 1999, 17, 19–49, doi:10.1146/annurev.immunol.17.1.19.
- Armitage, R.J.; Macduff, B.M.; Eisenman, J.; Paxton, R.; Grabstein, K.H. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J. Immunol. 1995, 154, 483–490.
- Waldmann, T.A.; Miljkovic, M.D.; Conlon, K.C. Interleukin-15 (dys)regulation of lymphoid homeostasis: Implications for therapy of autoimmunity and cancer. J. Exp. Med. 2020, 217, doi:10.1084/jem.20191062.
- Fehniger, T.A.; Caligiuri, M.A. Interleukin 15: Biology and relevance to human disease. Blood 2001, 97, 14–32, doi:10.1182/blood.v97.1.14.
- Tagaya, Y.; Kurys, G.; Thies, T.A.; Losi, J.M.; Azimi, N.; Hanover, J.A.; Bamford, R.N.; Waldmann, T.A. Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides. Proc. Natl. Acad. Sci. USA 1997, 94, 14444–14449, doi:10.1073/pnas.94.26.14444.
- Tan, X.; Lefrançois, L. Novel IL-15 isoforms generated by alternative splicing are expressed in the intestinal epithelium. Genes Immun. 2006, 7, 407–416, doi:10.1038/sj.gene.6364314.
- Gaggero, A.; Azzarone, B.; Andrei, C.; Mishal, Z.; Meazza, R.; Zappia, E.; Rubartelli, A.; Ferrini, S. Differential intracellular trafficking, secretion and endosomal localization of two IL-15 isoforms. Eur. J. Immunol. 1999, 29, 1265–1274, doi:10.1002/(sici)1521-4141(199904)29:04<1265::Aid-immu1265>3.0.Co;2-v.
- Waldmann, T.A. The Shared and Contrasting Roles of IL2 and IL15 in the Life and Death of Normal and Neoplastic Lymphocytes: Implications for Cancer Therapy. Cancer Immunol. Res. 2015, 3, 219–227, doi:10.1158/2326-6066.CIR-15-0009.
- Cao, X.; Kozak, C.A.; Liu, Y.J.; Noguchi, M.; O’Connell, E.; Leonard, W.J. Characterization of cDNAs encoding the murine interleukin 2 receptor (IL-2R) gamma chain: Chromosomal mapping and tissue specificity of IL-2R gamma chain expression. Proc. Natl. Acad. Sci. USA 1993, 90, 8464–8468, doi:10.1073/pnas.90.18.8464.
- Gruss, H.J.; Scott, C.; Rollins, B.J.; Brach, M.A.; Herrmann, F. Human fibroblasts express functional IL-2 receptors formed by the IL-2R alpha- and beta-chain subunits: Association of IL-2 binding with secretion of the monocyte chemoattractant protein-1. J. Immunol. 1996, 157, 851–857.
- Lin, J.X.; Leonard, W.J. The immediate-early gene product Egr-1 regulates the human interleukin-2 receptor beta-chain promoter through noncanonical Egr and Sp1 binding sites. Mol. Cell. Biol. 1997, 17, 3714–3722, doi:10.1128/mcb.17.7.3714.
- Ramirez, K.; Chandler, K.J.; Spaulding, C.; Zandi, S.; Sigvardsson, M.; Graves, B.J.; Kee, B.L. Gene deregulation and chronic activation in natural killer cells deficient in the transcription factor ETS1. Immunity 2012, 36, 921–932, doi:10.1016/j.immuni.2012.04.006.
- Sowell, R.T.; Goldufsky, J.W.; Rogozinska, M.; Quiles, Z.; Cao, Y.; Castillo, E.F.; Finnegan, A.; Marzo, A.L. IL-15 Complexes Induce Migration of Resting Memory CD8 T Cells into Mucosal Tissues. J. Immunol. 2017, 199, 2536–2546, doi:10.4049/jimmunol.1501638.
- Hong, C.; Luckey, M.A.; Ligons, D.L.; Waickman, A.T.; Park, J.Y.; Kim, G.Y.; Keller, H.R.; Etzensperger, R.; Tai, X.; Lazarevic, V.; et al. Activated T cells secrete an alternatively spliced form of common γ-chain that inhibits cytokine signaling and exacerbates inflammation. Immunity 2014, 40, 910–923, doi:10.1016/j.immuni.2014.04.020.
- Gesbert, F.; Malardé, V.; Dautry-Varsat, A. Ubiquitination of the common cytokine receptor gammac and regulation of expression by an ubiquitination/deubiquitination machinery. Biochem. Biophys. Res. Commun. 2005, 334, 474–480, doi:10.1016/j.bbrc.2005.06.121.
- Driesen, J.; Popov, A.; Schultze, J.L. CD25 as an immune regulatory molecule expressed on myeloid dendritic cells. Immunobiology 2008, 213, 849–858, doi:10.1016/j.imbio.2008.07.026.
- Nakamura, Y.; Russell, S.M.; Mess, S.A.; Friedmann, M.; Erdos, M.; Francois, C.; Jacques, Y.; Adelstein, S.; Leonard, W.J. Heterodimerization of the IL-2 receptor beta- and gamma-chain cytoplasmic domains is required for signalling. Nature 1994, 369, 330–333, doi:10.1038/369330a0.
- Takeshita, T.; Asao, H.; Ohtani, K.; Ishii, N.; Kumaki, S.; Tanaka, N.; Munakata, H.; Nakamura, M.; Sugamura, K. Cloning of the gamma chain of the human IL-2 receptor. Science 1992, 257, 379–382, doi:10.1126/science.1631559.
- Rubin, L.A.; Nelson, D.L. The soluble interleukin-2 receptor: Biology, function, and clinical application. Ann. Intern. Med. 1990, 113, 619–627, doi:10.7326/0003-4819-113-8-619.
- Murakami, S. Soluble interleukin-2 receptor in cancer. Front. Biosci. 2004, 9, 3085–3090, doi:10.2741/1461.
- Karim, A.F.; Eurelings, L.E.M.; Bansie, R.D.; van Hagen, P.M.; van Laar, J.A.M.; Dik, W.A. Soluble Interleukin-2 Receptor: A Potential Marker for Monitoring Disease Activity in IgG4-Related Disease. Mediat. Inflamm. 2018, 2018, 6103064, doi:10.1155/2018/6103064.
- Waldmann, T.A. The IL-2/IL-15 receptor systems: Targets for immunotherapy. J. Clin. Immunol. 2002, 22, 51–56, doi:10.1023/a:1014416616687.
- Ring, A.M.; Lin, J.-X.; Feng, D.; Mitra, S.; Rickert, M.; Bowman, G.R.; Pande, V.S.; Li, P.; Moraga, I.; Spolski, R.; et al. Mechanistic and structural insight into the functional dichotomy between IL-2 and IL-15. Nat. Immunol. 2012, 13, 1187–1195, doi:10.1038/ni.2449.
- Lodolce, J.P.; Burkett, P.R.; Boone, D.L.; Chien, M.; Ma, A. T cell-independent interleukin 15Ralpha signals are required for bystander proliferation. J. Exp. Med. 2001, 194, 1187–1194, doi:10.1084/jem.194.8.1187.
- Dubois, S.; Mariner, J.; Waldmann, T.A.; Tagaya, Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity 2002, 17, 537–547, doi:10.1016/s1074-7613(02)00429-6.
- Wuest, S.C.; Edwan, J.H.; Martin, J.F.; Han, S.; Perry, J.S.; Cartagena, C.M.; Matsuura, E.; Maric, D.; Waldmann, T.A.; Bielekova, B. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat. Med. 2011, 17, 604–609, doi:10.1038/nm.2365.
- Friedmann, M.C.; Migone, T.S.; Russell, S.M.; Leonard, W.J. Different interleukin 2 receptor beta-chain tyrosines couple to at least two signaling pathways and synergistically mediate interleukin 2-induced proliferation. Proc. Natl. Acad. Sci. USA 1996, 93, 2077–2082, doi:10.1073/pnas.93.5.2077.
- Lin, J.X.; Li, P.; Liu, D.; Jin, H.T.; He, J.; Ata Ur Rasheed, M.; Rochman, Y.; Wang, L.; Cui, K.; Liu, C.; et al. Critical Role of STAT5 transcription factor tetramerization for cytokine responses and normal immune function. Immunity 2012, 36, 586–599, doi:10.1016/j.immuni.2012.02.017.
- Lin, J.X.; Du, N.; Li, P.; Kazemian, M.; Gebregiorgis, T.; Spolski, R.; Leonard, W.J. Critical functions for STAT5 tetramers in the maturation and survival of natural killer cells. Nat. Commun. 2017, 8, 1320, doi:10.1038/s41467-017-01477-5.
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433, doi:10.1146/annurev-immunol-042617-053352.
- Miyazaki, T.; Liu, Z.J.; Kawahara, A.; Minami, Y.; Yamada, K.; Tsujimoto, Y.; Barsoumian, E.L.; Permutter, R.M.; Taniguchi, T. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell 1995, 81, 223–231, doi:10.1016/0092-8674(95)90332-1.
- Ross, S.H.; Rollings, C.; Anderson, K.E.; Hawkins, P.T.; Stephens, L.R.; Cantrell, D.A. Phosphoproteomic Analyses of Interleukin 2 Signaling Reveal Integrated JAK Kinase-Dependent and -Independent Networks in CD8(+) T Cells. Immunity 2016, 45, 685–700, doi:10.1016/j.immuni.2016.07.022.
- Pereno, R.; Giron-Michel, J.; Gaggero, A.; Cazes, E.; Meazza, R.; Monetti, M.; Monaco, E.; Mishal, Z.; Jasmin, C.; Indiveri, F.; et al. IL-15/IL-15Ralpha intracellular trafficking in human melanoma cells and signal transduction through the IL-15Ralpha. Oncogene 2000, 19, 5153–5162, doi:10.1038/sj.onc.1203873.
- Keating, N.; Nicholson, S.E. SOCS-mediated immunomodulation of natural killer cells. Cytokine 2019, 118, 64–70, doi:10.1016/j.cyto.2018.03.033.
- Delconte, R.B.; Kolesnik, T.B.; Dagley, L.F.; Rautela, J.; Shi, W.; Putz, E.M.; Stannard, K.; Zhang, J.G.; Teh, C.; Firth, M.; et al. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat. Immunol. 2016, 17, 816–824, doi:10.1038/ni.3470.
- Roediger, B.; Kyle, R.; Tay, S.S.; Mitchell, A.J.; Bolton, H.A.; Guy, T.V.; Tan, S.Y.; Forbes-Blom, E.; Tong, P.L.; Köller, Y.; et al. IL-2 is a critical regulator of group 2 innate lymphoid cell function during pulmonary inflammation. J. Allergy Clin. Immunol. 2015, 136, 1653–1663.e1657, doi:10.1016/j.jaci.2015.03.043.
- Robinette, M.L.; Bando, J.K.; Song, W.; Ulland, T.K.; Gilfillan, S.; Colonna, M. IL-15 sustains IL-7R-independent ILC2 and ILC3 development. Nat. Commun. 2017, 8, 14601, doi:10.1038/ncomms14601.
- Klose, C.S.; Blatz, K.; d’Hargues, Y.; Hernandez, P.P.; Kofoed-Nielsen, M.; Ripka, J.F.; Ebert, K.; Arnold, S.J.; Diefenbach, A.; Palmer, E.; et al. The transcription factor T-bet is induced by IL-15 and thymic agonist selection and controls CD8αα(+) intraepithelial lymphocyte development. Immunity 2014, 41, 230–243, doi:10.1016/j.immuni.2014.06.018.
- Lenardo, M.J. Fas and the art of lymphocyte maintenance. J. Exp. Med. 1996, 183, 721–724, doi:10.1084/jem.183.3.721.
- Zhang, X.; Sun, S.; Hwang, I.; Tough, D.F.; Sprent, J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 1998, 8, 591–599, doi:10.1016/s1074-7613(00)80564-6.
- Schluns, K.S.; Klonowski, K.D.; Lefrançois, L. Transregulation of memory CD8 T-cell proliferation by IL-15Ralpha+ bone marrow-derived cells. Blood 2004, 103, 988–994, doi:10.1182/blood-2003-08-2814.
- Vang, K.B.; Yang, J.; Mahmud, S.A.; Burchill, M.A.; Vegoe, A.L.; Farrar, M.A. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J. Immunol. 2008, 181, 3285–3290, doi:10.4049/jimmunol.181.5.3285.
- Burchill, M.A.; Yang, J.; Vogtenhuber, C.; Blazar, B.R.; Farrar, M.A. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 2007, 178, 280–290, doi:10.4049/jimmunol.178.1.280.
- Fontenot, J.D.; Rasmussen, J.P.; Gavin, M.A.; Rudensky, A.Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005, 6, 1142–1151, doi:10.1038/ni1263.
- Li, M.O.; Rudensky, A.Y. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat. Rev. Immunol. 2016, 16, 220–233, doi:10.1038/nri.2016.26.
- Boyman, O.; Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 2012, 12, 180–190, doi:10.1038/nri3156.
- Hirakawa, M.; Matos, T.R.; Liu, H.; Koreth, J.; Kim, H.T.; Paul, N.E.; Murase, K.; Whangbo, J.; Alho, A.C.; Nikiforow, S.; et al. Low-dose IL-2 selectively activates subsets of CD4(+) Tregs and NK cells. JCI Insight 2016, 1, e89278, doi:10.1172/jci.insight.89278.
- Zhao, H.; Nguyen, H.; Kang, J. Interleukin 15 controls the generation of the restricted T cell receptor repertoire of gamma delta intestinal intraepithelial lymphocytes. Nat. Immunol. 2005, 6, 1263–1271, doi:10.1038/ni1267.
- Van Acker, H.H.; Campillo-Davo, D.; Roex, G.; Versteven, M.; Smits, E.L.; Van Tendeloo, V.F. The role of the common gamma-chain family cytokines in γδ T cell-based anti-cancer immunotherapy. Cytokine Growth Factor Rev. 2018, 41, 54–64, doi:10.1016/j.cytogfr.2018.05.002.
- Leclercq, G.; Debacker, V.; de Smedt, M.; Plum, J. Differential effects of interleukin-15 and interleukin-2 on differentiation of bipotential T/natural killer progenitor cells. J. Exp. Med. 1996, 184, 325–336, doi:10.1084/jem.184.2.325.
- Arneja, A.; Johnson, H.; Gabrovsek, L.; Lauffenburger, D.A.; White, F.M. Qualitatively different T cell phenotypic responses to IL-2 versus IL-15 are unified by identical dependences on receptor signal strength and duration. J. Immunol. 2014, 192, 123–135, doi:10.4049/jimmunol.1302291.
- Leonard, W.J. Cytokines and immunodeficiency diseases. Nat. Rev. Immunol. 2001, 1, 200–208, doi:10.1038/35105066.
- Sadlack, B.; Löhler, J.; Schorle, H.; Klebb, G.; Haber, H.; Sickel, E.; Noelle, R.J.; Horak, I. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur. J. Immunol. 1995, 25, 3053–3059, doi:10.1002/eji.1830251111.
- Sadlack, B.; Merz, H.; Schorle, H.; Schimpl, A.; Feller, A.C.; Horak, I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 1993, 75, 253–261, doi:10.1016/0092-8674(93)80067-o.
- Schorle, H.; Holtschke, T.; Hünig, T.; Schimpl, A.; Horak, I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature 1991, 352, 621–624, doi:10.1038/352621a0.
- Willerford, D.M.; Chen, J.; Ferry, J.A.; Davidson, L.; Ma, A.; Alt, F.W. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity 1995, 3, 521–530, doi:10.1016/1074-7613(95)90180-9.
- Lodolce, J.P.; Boone, D.L.; Chai, S.; Swain, R.E.; Dassopoulos, T.; Trettin, S.; Ma, A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 1998, 9, 669–676, doi:10.1016/s1074-7613(00)80664-0.
- Kennedy, M.K.; Glaccum, M.; Brown, S.N.; Butz, E.A.; Viney, J.L.; Embers, M.; Matsuki, N.; Charrier, K.; Sedger, L.; Willis, C.R.; et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 2000, 191, 771–780, doi:10.1084/jem.191.5.771.
- Cornish, G.H.; Sinclair, L.V.; Cantrell, D.A. Differential regulation of T-cell growth by IL-2 and IL-15. Blood 2006, 108, 600–608, doi:10.1182/blood-2005-12-4827.
- Klebanoff, C.A.; Finkelstein, S.E.; Surman, D.R.; Lichtman, M.K.; Gattinoni, L.; Theoret, M.R.; Grewal, N.; Spiess, P.J.; Antony, P.A.; Palmer, D.C.; et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc. Natl. Acad. Sci. USA 2004, 101, 1969–1974, doi:10.1073/pnas.0307298101.
- Beltra, J.C.; Bourbonnais, S.; Bédard, N.; Charpentier, T.; Boulangé, M.; Michaud, E.; Boufaied, I.; Bruneau, J.; Shoukry, N.H.; Lamarre, A.; et al. IL2Rβ-dependent signals drive terminal exhaustion and suppress memory development during chronic viral infection. Proc. Natl. Acad. Sci. USA 2016, 113, E5444–E5453, doi:10.1073/pnas.1604256113.
- Heon, E.K.; Wulan, H.; Macdonald, L.P.; Malek, A.O.; Braunstein, G.H.; Eaves, C.G.; Schattner, M.D.; Allen, P.M.; Alexander, M.O.; Hawkins, C.A.; et al. IL-15 induces strong but short-lived tumor-infiltrating CD8 T cell responses through the regulation of Tim-3 in breast cancer. Biochem. Biophys. Res. Commun. 2015, 464, 360–366, doi:10.1016/j.bbrc.2015.06.162.
- Wagner, J.A.; Rosario, M.; Romee, R.; Berrien-Elliott, M.M.; Schneider, S.E.; Leong, J.W.; Sullivan, R.P.; Jewell, B.A.; Becker-Hapak, M.; Schappe, T.; et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J. Clin. Investig. 2017, 127, 4042–4058, doi:10.1172/jci90387.
- Marçais, A.; Cherfils-Vicini, J.; Viant, C.; Degouve, S.; Viel, S.; Fenis, A.; Rabilloud, J.; Mayol, K.; Tavares, A.; Bienvenu, J.; et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat. Immunol. 2014, 15, 749–757, doi:10.1038/ni.2936.
- Mao, Y.; van Hoef, V.; Zhang, X.; Wennerberg, E.; Lorent, J.; Witt, K.; Masvidal, L.; Liang, S.; Murray, S.; Larsson, O.; et al. IL-15 activates mTOR and primes stress-activated gene expression leading to prolonged antitumor capacity of NK cells. Blood 2016, 128, 1475–1489, doi:10.1182/blood-2016-02-698027.
- Felices, M.; Lenvik, A.J.; McElmurry, R.; Chu, S.; Hinderlie, P.; Bendzick, L.; Geller, M.A.; Tolar, J.; Blazar, B.R.; Miller, J.S. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight 2018, 3, doi:10.1172/jci.insight.96219.
- Ndhlovu, L.C.; Lopez-Vergès, S.; Barbour, J.D.; Jones, R.B.; Jha, A.R.; Long, B.R.; Schoeffler, E.C.; Fujita, T.; Nixon, D.F.; Lanier, L.L. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012, 119, 3734–3743, doi:10.1182/blood-2011-11-392951.
- Viel, S.; Marcais, A.; Guimaraes, F.S.; Loftus, R.; Rabilloud, J.; Grau, M.; Degouve, S.; Djebali, S.; Sanlaville, A.; Charrier, E.; et al. TGF-beta inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci. Signal. 2016, 9, ra19, doi:10.1126/scisignal.aad1884.