Terephthalate (PET) is one of the most significant modern plastics to be invented. PET is a semi-crystalline polymer, with mechanical properties that depend on crystallinity level. This material is considered to be a thermoplastic polyester material, which is now used globally.
- asphalt binder
- polyethylene terephthalate
1. Introduction
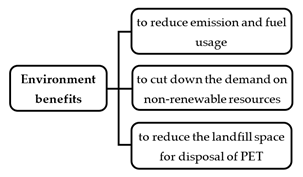
These days, there are tons of PET waste products that are generated around the world. This waste presents a real environmental hazard because PET is not biodegradable [20]. Currently, modern techniques to dispose of PET waste and polymers include waste landfills, open burning, and recycling. However, these techniques do not help environmental protection efforts. The waste landfill is the simplest and oldest waste disposal strategy in the world, but it has led to many issues such as land occupation, groundwater pollution, unsafe disposal, and wastage of resources. Hence, reprocessing (recycling) these plastic materials seems to be the best option. Recycling is a convincing and logical strategy for reusing PET waste. Nevertheless, recycling is essentially limited due to its high cost [21]. As shown in Figure 2, there are three specific environmental advantages of using PET in new pavement construction.
Figure 2. Environmental benefits of using PET for new pavement construction.
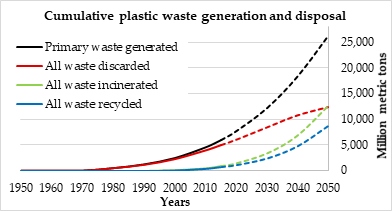
Figure 3 illustrates the accumulated quantity of the generated plastic waste and disposed 1950 to 2015 and the expected quantity by 2050. Almost 16% of this quantity was recycled up to 2015. It is predicted that up to 33% of the waste plastic will be recycled by 2050. Even if this prediction becomes true, the quantity of unrecycled plastic waste would leave much to be desired [1].
Figure 3. The accumulated quantity of the generated plastic waste and Disposed 1950 to 2015 and the expected quantity by 2050 [1].
2. Methods for Recycling Polyethylene Terephthalate Waste
The most practical methods to recycle Polyethylene Terephthalate are mechanical and chemical. The mechanical recycling of PET will decrease the quality of the recycled PET because it involves adhesive contaminants. Additionally, the chemical recycling of PET has a higher cost than mechanical recycling because the former must use chemicals such as catalysts at a certain temperature and at high pressure [22]. Mechanical recycling is also termed physical recycling; this process re-processes PET material by melting extrusions of the material after separating them from their integrated contaminants. It mostly involves the following processes and treatments: (1) insulating the PET from other plastic materials, (2) washing the filth and other contaminants followed by drying, (3) grinding the PET particles to reduce the particle size before reuse, (4) Heat extrusion, and (5) shaping and grinding the PET products. Physical recycling is simpler to apply and generally requires lesser effort than chemical recycling [23,24].
Chemical recycling is an alternative to the physical recycling method. This method chemically polymerises or degrades PET waste before re-use. Many chemical recycling chemical processes can be used, such as hydrogenation, glycolysis, aminolysis, methanolysis, and hydrolysis [17,24]. Depending on the chemical depolymerisation process of PET post-consumer, the corresponding terephthalic acid (TPA) or ethylene glycol can be produced from the yield resulting from aminolytic chain cleavage [17,25]. The depolymerisation of PET waste material can be accomplished using several types of amines, for example, polyamines, ethanolamine, allylamine, and tri-ethanol amine. The aminolysis response is applied to manufacture PET products such as plasticisers and rigid polyurethane foams [17,26].
3. Physical and Chemical Properties of PET
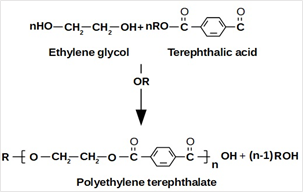
Virgin PET is a superior material for certain applications. This material has several excellent characteristics, such as good tensile strength, reasonable thermal stability, chemical resistance, processing capability, color capability, and clarity [27]. It can be formed by pressures and at normal temperatures. PET can be created from petroleum hydrocarbons, from the reaction between ethylene glycol and Terephthalate acid, as shown in Figure 4 [28]. The basic physical and chemical properties of commercially-used PET plastics are indicated in Table 1 [27].
Figure 4. PET produced from the reaction between ethylene glycol and terephthalic acid.
Table 1. Physical and chemical properties of PET [27].
|
Property |
Test Method |
Value (Unit) |
|
Molecular weight (of repeating unit) |
˗ |
192 ( ) |
|
Mark–Houwink parameters |
˗ |
k = 3.72 × ( ) a = 0.73 |
|
Weight-average MW |
˗ |
30,000–80,000 (g ) |
|
Density |
˗ |
1.41 (g ) |
|
Glass transition temperature |
(DSC) 1 |
69–115 (°C) |
|
Melting temperature |
(DSC) 1 |
265 (°C) |
|
Heat of fusion |
(DSC) 1 |
166 (J/g) |
|
Breaking strength |
Tensile |
50 (MPa) |
|
Tensile strength (Young’s modulus) |
˗ |
1700 (MPa) |
|
Yield strain |
Tensile |
4 (%) |
|
Impact strength |
ASTM D256-86 |
90 ( ) |
|
Water absorption (after 24 h) |
˗ |
0.5 (%) |
1 Differential scanning calorimetry (DSC).
4. Performance of PET Modified Asphalt Binder
Researchers have investigated the benefits of using waste materials in the construction industry, to better manage waste and to enhance the quality of construction materials [29,30]. Currently, several waste materials, such as waste glass, plastic, tires, etc., are used to improve the construction of various pavement layers, including the asphalt surface [31]. Asphalt pavements will be exposed to deterioration after construction, due to traffic loading activity and climatic conditions. This process can be delayed if good materials are used in the construction phase. Usually, materials that used as a modifier of asphalt binder have better performance than conventional ones [32]. According to Mashaan et al. [33], asphalt binder modification provides several benefits such as
- A sufficient increase in the consistency of the asphalt pavement to prevent plastic deformation at high temperatures;
- Enhanced asphalt binder elasticity and flexibility to avoid loss due to chipping or cracking at low temperature;
- Increased adhesion between asphalt binder and aggregate; improved ageing resistance and homogeneity, and high thermal stability, which helps decrease the stiffening and initial ageing of asphalt binders during mixing and road construction.
Recycled PET can be used as an improved modifier for asphalt binders. This application could be counted as a method to reuse PET waste and in turn, addresses potential environmental risks and reduces construction costs that would result if polymers were used in the asphalt mixtures [23,34,35]. Moreover, the positive effects of PET have been proven, such as providing high-temperature characteristics for the asphalt mixture [36]. Nevertheless, PET is not always effective at increasing asphalt binder elasticity, especially during dramatic and unexpected drops in temperature. This condition degrades the intermediate- and low-temperature characteristics of the asphalt binder [35]. In order to investigate the impact of PET on asphalt binder properties, PET was blended with the asphalt binder. The progressive addition of PET has been shown enhancing in the consistency of the binder and increased its elastic behavior. However, the increase in viscosity of modified binder leads to higher mixing and compaction temperatures. Although this is not economic in the short term, it can produce roads with less maintenance requirements. The addition of PET has reduced modified asphalt binder oxidation and has proved to be promising in anti-ageing action [37].
This entry is adapted from the peer-reviewed paper 10.3390/su13031303