Neuroendocrine neoplasms are rare entities consisting of a heterogeneous group of tumors that can originate from neuroendocrine cells present in the whole body. Their different behavior, metastatic potential, and prognosis are highly variable, depending on site of origin, grade of differentiation, and proliferative index. The aim of our work is to summarize the current knowledge of immunotherapy in different neuroendocrine neoplasms and its implication in clinical practice.
- neuroendocrine tumors
- immunotherapy
- neuroendocrine neoplasia
- neuroendocrine carcinoma
- immune checkpoint inhibitors
- Merkel cell carcinoma
- small cell lung cancer
1. Introduction
Neuroendocrine neoplasias (NENs) are rare tumors, but their incidence has increased in the last few years and is estimated to be around 7 cases per 100,000 [1]. NENs are a heterogeneous group of tumors that can originate from neuroendocrine cells present in the whole body. The most frequent origins are the gastroenteropancreatic (GEP) system (around 70% of cases) and lungs (around 20% of cases) [1–3]. Less common are NENs originating from the genito-urinary tract, female gynecological system, and Merkel cells in the skin. Their behavior, metastatic potential, and prognosis are highly variable, depending on site of origin, grade of differentiation, and proliferative index.
GEP–NENs are graded according to the Ki67 proliferation index and grouped into Grades 1 (G1, Ki67 <3%), 2 (G2, Ki67 3–20%), and 3 (G3, Ki67 >20%), following the World Health Organization (WHO) 2010 classification. In this classification, all G3 tumors were referred to as neuroendocrine carcinomas (NECs) and were characterized by aggressive behavior. However, the WHO 2017 classification introduced the category of well-differentiated neuroendocrine tumors (NETs) with Ki67 ≥20% (G3) among pancreatic NENs, with clinical and morphological characteristics halfway between moderately differentiated NETs and NECs [2]. The WHO 2019 classification extended this concept to all gastrointestinal NENs.
Differently, NENs of pulmonary origin are grouped into low-grade (typical carcinoid (TC)), intermediate-grade (atypical carcinoid (AC)), and high-grade (small cell lung cancer (SCLC) and large cell neuroendocrine carcinoma (LCNEC)) [3,4]. This classification reflects the increasing biological aggressiveness and worsening prognosis from TC/AC to SCLC/LCNEC, while TC and AC are considered to have similar behavior, as well as LCNEC and SCLC. NENs show several molecular similarities, irrespective of the site of origin, but different classifications are used depending on the organ of origin [5]. In order to harmonize NEN classification, the International Agency for Research on Cancer (IARC), together with WHO, proposed a new uniform classification system for NENs across different sites. It distinguishes between differentiated NETs and poorly differentiated NECs, supported by morphological, histologic, epidemiologic, genetic, and prognostic differences at specific anatomic sites [6,7].
Merkel cell carcinoma (MCC) is a rare and aggressive NEN of the skin that typically occurs in older patients with a history of sun exposure and can affect patients with an immunodeficiency state. The oncogenic Merkel cell polyomavirus (MCPyV) can be found in 80% of the MCCs [8,9].
The therapeutic approach to NENs is very different and is based on the location of the primary lesion, the morphological differentiation, and the grading. For advanced unresectable low- and intermediate-grade NENs, mainstays of treatment are somatostatin analog (SSA), tyrosine kinase inhibitors (TKIs), mammalian target of rapamycin (mTOR) inhibitors, and peptide receptor radionuclide therapy (PRRT). On the contrary, the backbone of the treatment of advanced high-grade and poorly-differentiated NECs is chemotherapy.
2. Immunotherapy in Human Cancers and Rationale in NENs
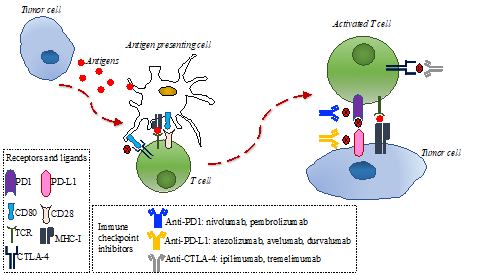
The immune response is directed by the balance between stimulating and inhibitory signals that regulate the action and proliferation of immune cells. The anti-tumor immune response is modulated by the interaction of several proteins located on the membrane of T-cells and antigen-presenting cells (APC), referred to as immune checkpoints [7]. Tumors can escape the immune system recognition through expression ligands that interact with immune checkpoints expressed on T-cells, such as cytotoxic T lymphocyte-associated protein-4 (CTLA-4) and programmed cell death 1 (PD-1) [10,11]. Therefore, in recent years, different types of immune checkpoint inhibitors, consisting of monoclonal antibodies targeting CTLA-4 or PD-1 on T-cell or programmed cell death ligand 1 (PD-L1) on tumor cells, were successfully tested in several tumors (Figure 1) and changed clinical practice, given the improvement in patients’ outcomes [12–36].
Figure 1. Immune response to tumor cells and main mechanisms of action of anti-programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) and anti-cytotoxic T lymphocyte-associated protein-4 (CTLA-4). Tumor cells release antigens that are uptaken by antigen-presenting cells. These cells present tumoral antigens to naïve T-cells, thus activating them. The interaction between PD-1 in activated T-cells and PD-L1 in tumor cells can inhibit the immune response. CD80 on antigen-presenting cells can bind to CTLA-4 on activated T-cells and inhibit the immune response. Anti PD-1/PD-L1 and anti-CTLA4 monoclonal antibodies can bind to PD-1 in activated T-cells, PD-1 in tumor cells, or CTLA-4 in tumor cells, respectively, thus restoring the immune response.
However, there is still limited evidence of the efficacy of immune checkpoint inhibition in many types of NENs, while it proved effective in other difficult-to-treat NENs, such as SCLC and MCC [20,22,23,37].
3. Predictive Biomarkers for Immunotherapy
As the therapeutic scenario for NENs changes with the addition of immunotherapy, so does the need to find predictive biomarkers that can guide clinical decisions. Since benefit on immunotherapy treatments is usually limited to a subset of patients, great effort has been made by the research community to find predictive factors able to identify such patients. Studies in commonly immunotherapy-treated tumors, such as malignant melanoma and non-small cell lung cancer, have identified biomarkers that might have the potential to predict response to immunotherapy. In particular, high levels of PD-L1 expression and a high tumor mutational burden (TMB), defined as the number of non-synonymous mutations per megabase of sequenced genome, were associated with an increased benefit to immunotherapy [38–40]. However, durable responses to immunotherapy also occur in patients whose tumors have low or no PD-L1 expression and low TMB so that using only these two biomarkers could exclude potential responders from treatment [39,40].
While the higher PD-L1 expression is intuitively linked to the mechanism of action of immune checkpoint inhibitors, the role of TMB is still under debate. It is likely that a higher number of somatic mutations increase the likelihood of generating neoantigens that can be immunogenic and recognized by T-cells to trigger an immune response [40].
With regard to neuroendocrine tumors, most evidence comes from SCLC. The rationale for the use of immunotherapy in this setting derives from the fact that SCLC has one of the highest TMB among human cancers (median 8 mutations per megabase (mut/Mb)), despite expressing PD-L1 in only 20% of the cases [41–43]. However, TMB alone does not completely predict benefit from immune checkpoint inhibition and should be integrated with other pathological and genetic factors in additional models to improve biomarker performances [44].
There are few studies supporting the use of immunotherapy in GEP–NENs. Expression of PD-L1 and PD-1 is associated with higher-grade tumors (i.e., NET G3 and NECs) and with worse progression-free survival (PFS) and overall survival (OS) [45–48]. A recent work on NETs of different grades and primary sites, including pancreas, midgut and lung, analyzed expression characteristics of PD-L1, PD-L2, indoleamine-deoxygenase-1 (IDO-1), tumor-infiltrating T-lymphocytes (TILs), as well as biomarkers of hypoxia and angiogenesis [49]. Among 102 NET samples, PD-L1 expression was highest in lung NETs, and lowest in ileal NETs, while PD-L2 expression was highest in pancreatic NETs. Furthermore, exhausted and regulatory TILs were enriched in PD-L1-positive NETs but decreased in G3 well-differentiated NETs. This suggests that immune tolerance in NETs might be driven by PD-L1/2 expression and that NETs that express PD-L1 and with TILs might benefit from PD-L1 inhibition. Microsatellite instability (MSI) is a tumor-agnostic marker of response to PD-1/PD-L1 blockade [29] and can be found in GEP mixed adeno-neuroendocrine carcinoma (MANEC), while it is rare in G1-G2 GEP-NET [50,51]. Furthermore, TMB is typically low in G1–G2 NENs, while it is higher in NECs, as reported in a study that used 17 mut/Mb as a cut-off to define high and low TMB tumors [52,53].
Given the urgent need for identifying reliable predictive factors, several ongoing clinical trials include a biomarker analysis to identify factors able to predict for response to immunotherapy (e.g., NCT03095274, NCT03074513, NCT03728361). The latter trial (NCT03728361) investigates the efficacy of the combination of nivolumab and temozolomide, an alkylating agent, in NET of any grade, NEC, and SCLC. Among the exploratory objectives of this study, there are to be determined whether baseline TMB is predictive for response to therapy in SCLC patients, whether changes in blood-based TMB during treatment may predict clinical benefit in the whole population, and whether a composite immune- and tumor-cell-staining score can be developed, with or without PD-L1 by immunohistochemistry, to predict response in the SCLC cohort.
This entry is adapted from the peer-reviewed paper 10.3390/cancers12040832