In recent years, considerable importance is given to the use of agrifood wastes as they contain several groups of substances that are useful for development of functional foods. As muscle foods are prone to lipid and protein oxidation and perishable in nature, the industry is in constant search of synthetic free additives that help in retarding the oxidation process, leading to the development of healthier and shelf stable products.
- pomegranate
- bioactive compounds
- lipid and protein oxidation
- meat
- fish
- shelf life
Note:All the information in this draft can be edited by authors. And the entry will be online only after the authors edit and submit it.
1. Introduction
Muscle foods, in particular meat and fish, are considered excellent sources of high-quality proteins containing balanced amino acids, vitamins (B group), minerals, and a number of other nutrients [1–4]. Even though having high nutrient contents, muscle foods also contain metal catalysts, haem pigment, various oxidizing agents, and abundant unsaturated fatty acids, which are unstable, especially when exposed to extreme environmental conditions such as constant high temperature, air, and light [5]. These food products with high water content and moderate pH are perishable in nature, hence cannot be stored for longer periods without any preservatives [6]. The susceptibility of these products to spoilage results from microbial activities and undesirable chemical changes, such as oxidation of muscle proteins and lipids during storage [7–9]. Lipid oxidation of muscle foods results in an extensive color loss, structural damage to protein, and production of rancid or unpleasant flavors [4,10,11]. These changes negatively affect the sensory quality, nutritional value, and consumer acceptability, and consequently shorten the shelf life of muscle foods [12–17]. As far as protein oxidation in muscle foods is concerned, it changes the amino acid structures, leading to the formation of carbonyl and reduction of sulfhydryl content [18]. Oxidative changes ultimately affect the tenderness and water holding capacity of muscle food during storage [19,20].
There are different ways to prevent the microbial activities and oxidative deterioration of muscle foods. Synthetic chemicals are mostly used to inhibit such types of changes and minimize the formation of toxic compounds such as cholesterol oxidation products [21,22]. However, in recent times, natural preservatives extracted from various agrifood wastes are being explored by food processors that not only contain antimicrobials and antioxidants but also are abundant, cheap, and environment friendly. In addition, many plant parts (fruits, roots, bark, leaves) and their coproducts are also reported to provide a rich source of natural bioactive compounds (polyphenolic, dietary fibre, and flavonoids) that not only play a vital role in inhibiting oxidative changes (antioxidants) but also help in suppressing microbial growth (antimicrobial), thereby preventing several diseases [23,24]. Again, consumers worldwide also prefer these natural preservatives, which are considered safe and exert positive health effects over synthetic chemicals that have toxicity and health risks [5,25,26].
Pomegranate (Punica granatum L.), a member of the family Punicaceae, is a deciduous shrub or small tree widely cultivated in the Middle East, European, and Southeast Asian countries [5]. Each and every part of the pomegranate plant (leaves, stem, fruits, bark and roots) possess numerous bioactive compounds like phenolic compounds, including hydrolyzable tannins (pedunculagin, punicalin, punicalagin, and ellagic and gallic acids), flavonoids (catechins, anthocyanins, and other complex flavonoids) and complex polysaccharides [27,28]. It is a fruit, commonly known as ‘‘seeded apple’’ or ‘‘granular apple’’, highly valued and consumed worldwide for its pleasant taste, nutritional values, and medicinal properties [29]. Pomegranate fruit is used in the fruit processing and beverage industry for the preparation of juice and soft drinks, and during the production process, a large quantity of fruit-derived low-cost nonedible waste (mostly peel and seed) is generated. These wastes are valuable sources of bioactive compounds and could either be used as functional food ingredients or as food additives, nutraceuticals, and supplements to enrich phenolic content in diets [29,30]. These bioactive compounds, apart from being natural, exert antioxidant and antimicrobial activity and are reported to improve the quality, safety, and extend the shelf life of different types of food products such as oils [5], meat [23,31,32,33], fish [34,35,36], and dairy products like cheese, curd, fermented milk [37], cereal-based cookies [38].
2. Pomegranate Fruit and Its By-products
Pomegranate fruit, regarded as superfruit of the next generation, is quite popular throughout the globe due to its sweetness, acidic juices, and extensive medicinal properties, including antimicrobial, antioxidant, antimutagenic, antihypertensive, and hepatoprotective properties [37,39,40]. The outer hard covering of the fruit is red-purple in colour and called the pericarp, whereas the inner spongy wall is called mesocarp (white “albedo”), where seeds are attached. A mature pomegranate fruit measures about 6–10 cm in diameter, weighs 200 g on an average and usually contains 50% peel, 40% arils and 10% seeds. Further, the pomegranate fruit processing establishments also generate a large quantity of by-products/wastes after the extraction of juice from the fruits. The wastes are called pomace or bagasse, which is nothing but a mixture of peel, seed, and mesocarp, which remains underutilized in the food industry. These wastes could be fortified in various food systems for the design and development of healthy functional foods with improved quality and shelf life, offering health benefits [41–43]. The fruit is a good source of dietary fibre (both soluble and insoluble), but contains no cholesterol or saturated fats and is low in sugar. In addition, it also contains about 80–85 calories per 100 g, vital minerals (potassium, copper, manganese, and zinc), and vitamin C and B complex groups, such as pantothenic acid (vitamin B-5), folates, pyridoxine, and vitamin K [44]. The proximate composition, major micronutrients, vitamins, and polyphenolic contents of pomegranate peel and seed powder are represented in Table 1.
Table 1. Proximate composition, major micronutrients, vitamins, and polyphenolic content of pomegranate peel and seed powder.
|
Parameters |
Pomegranate Peel |
Pomegranate Seed Powder |
|
Moisture (%) |
13.7 |
5.8 |
|
Protein (%) |
3.1 |
13.7 |
|
Fat (%) |
1.8 |
29.6 |
|
Ash (%) |
3.3 |
1.5 |
|
Fibre (%) |
11.2 |
39.4 |
|
Carbohydrates (%) |
80.5 |
13.5 |
|
Calcium (mg/100g) |
338.50 |
229.20 |
|
Potassium (mg/100g) |
146.40 |
434.40 |
|
Sodium (mg/100g) |
66.43 |
33.03 |
|
Phosphorus (mg/100g) |
117.90 |
481.10 |
|
Iron (mg/100g) |
5.93 |
10.88 |
|
Vitamin C (mg/100g) |
12.90 |
3.02 |
|
Vitamin E (mg/100g) |
3.99 |
1.35 |
|
Total polyphenol (mg/g GAE) |
53.65 (WE) |
7.94 (WE) |
|
85.60 (ME) |
11.84 (ME) |
|
|
Total flavonoids (mg/g TE) |
21.03 (WE) |
3.30 (WE) |
|
51.52 (ME) |
6.79 (ME) |
|
|
Total anthocyanins (mg/g CGE) |
51.02 (WE) |
19.62 (WE) |
|
102.02 (ME) |
40.84 (ME) |
|
|
Hydrolyzable tannins (mg/g TAE) |
62.71 (WE) |
32.86 (WE) |
|
139.63 (ME) |
29.57 (ME) |
GAE: gallic acid equivalents, TAE: tannic acid equivalents, CGE: cyanidin-3-glucoside equivalents, WE: water extract, ME: methanol extract. Source: [29,45,46].
3. Polyphenolic and Flavonoid Compounds in Pomegranate Fruit By-Products
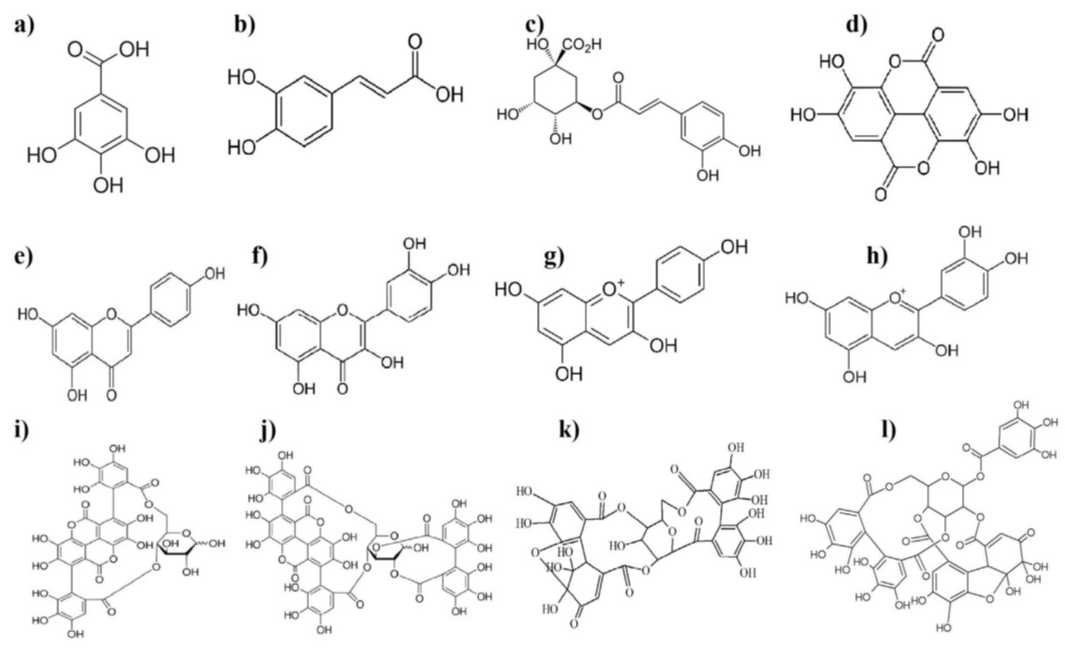
Polyphenols are a structural class of organic chemicals containing large multiples of phenolic structural units. The chemical structure of major phenolic compounds from pomegranate fruit and its by-products are presented in Figure 1, including ellagitannins (ellagic acid, punicalagin, gallic acid, punicalin), anthocyanins (cyanidin and pelargonidin), phenolic acids like caffeic acid, chlorogenic acid, and flavonoids (quercetin).
Figure 1. Chemical structure of major phenolic compounds from pomegranate fruit: (a) gallic acid, (b) caffeic acid, (c) chlorogenic acid, (d) ellagic acid, (e) apigenin, (f) quercetin, (g) pelargonidin, (h) cyanidin, (i) punicalin, (j) punicalagin, (k) granatin A, and (l) granatin B.
The phenolics and polyphenolic compounds in pomegranate fruit and its by-products (seed, juice, pomace, and peel) have been studied in detail by several workers. Not only the succulent testa of pomegranate fruit, but the peels are also sources of phenolics, pectin, and complex polysaccharides, whereas the arils are rich in water, sugars, organic acids, and polyphenolic compounds, especially flavonoids. In addition, the major phenolic compound in the pomegranate juice is anthocyanin, the pericarp, and mesocarp are sources of hydrolyzable tannins [37,47]. The by-products also contain important compounds and chemicals that are valuable sources of antioxidants, tannins, dynes, and alkaloids [29,48]. The major chemical constituents, phenolics, and organic compounds reported in different pomegranate plant parts are presented in Table 2.
Table 2. Pomegranate plant parts and their chemical constituents.
|
Pomegranate Plant Parts |
Chemical Constituents |
|
Pomegranate juice from the succulent testa |
Glucose, ascorbate, ellagic acid, anthocyanins, caffeic acid, catechin, quercetin, amino acids and minerals. |
|
Seed oil |
Punicic acid, ellagic acid, sterols, phytoestrogens |
|
Peel (pericarp) |
Phenolic punicalagins, gallic acid, catechin, flavones, etc.; Flavonoids (catechin, flavan-3-ol, epicatechin, quercetin, kaempferol, rutin, kaempferol 3-O-glycoside, kaempferol 3-O-rhamnoglycoside, naringin epigallocatechin 3-gallate, luteolin, and luteolin 7-O-glycoside); Ellagitannins (punicalagin, punicalin, corilagin, gallagyldilacton, tellimagrandin, casuarinin, pedunculagin, granatin A, and granatin B); Pelletierine alkaloids (pelletierine); caffeic acid; p-coumaric acid; chlorogenic acid; quinic acid; polyphenols (saponins, ellagic tannins, ellagic acid, and gallic acid); anthocyanidins; additionally, triterpenoids, steroids, glycosides, carbohydrate, vitamin C, ascorbic acid, and tannins |
|
Leaves |
Tannins, flavone glycosides, luteolin, apigenin |
|
Flowers |
Gallic acid, urosolic acid, triterpenoid compounds, including maslinic and asiatic acid |
|
Bark and roots |
Punicalin, punicalagin, piperidine alkaloids, ellagitannins |
Source: [29,45,46].
References
- Gálvez, F.; Maggiolino, A.; Domínguez, R.; Pateiro, M.; Gil, S.; De Palo, P.; Carballo, J.; Franco, D.; Lorenzo, J.M. Nutritional and meat quality characteristics of seven primal cuts from 9-month-old female veal calves: A preliminary study. Sci. Food Agric. 2019, 99, doi:10.1002/jsfa.9508.
- Gálvez, F.; Domínguez, R.; Maggiolino, A.; Pateiro, M.; Carballo, J.; De Palo, P.; Barba, F.J.; Lorenzo, J.M. Meat quality of commercial chickens rearing in different production systems: Industrial, range and organic. Anim. Sci. 2019, doi:10.2478/aoas-2019-0067.
- Gálvez, F.; Domínguez, R.; Pateiro, M.; Carballo, J.; Tomasevic, I.; Lorenzo, J.M. Effect of gender on breast and thigh turkey meat quality. Poult. Sci. 2018, doi:10.1080/00071668.2018.1465177.
- Das, A.K.; Das, A.; Nanda, P.K.; Madane, P.; Biswas, S.; Zhang, W.; Lorenzo, J.M. A comprehensive review on antioxidant dietary fibre enriched meat-based functional foods. Trends Food Sci. Technol. 2020, 99, 323–336, doi:10.1016/j.tifs.2020.03.010.
- El-Hadary, A.E.; Taha, M. Pomegranate peel methanolic-extract improves the shelf-life of edible-oils under accelerated oxidation conditions. Food Sci. Nutr. 2020, 8, 1798–1811, doi:10.1002/fsn3.1391.
- Das, A.K.; Nanda, P.K.; Bandyopadhyay, S.; Banerjee, R.; Biswas, S.; McClements, D.J. Application of nanoemulsion-based approaches for improving the quality and safety of muscle foods: A comprehensive review. Rev. Food Sci. Food Saf. 2020, 19, 2677–2700, doi:10.1111/1541-4337.12604.
- Das, A.K.; Nanda, P.K.; Das, A.; Biswas, S. Hazards and Safety Issues of Meat and Meat Products. Food Saf. Hum. Health 2019, 145–168, doi:10.1016/b978-0-12-816333-7.00006-0.
- Hygreeva, D.; Pandey, M.C. Novel approaches in improving the quality and safety aspects of processed meat products through high pressure processing technology—A review. Trends Food Sci. Technol. 2016, 54, 175–185, doi:10.1016/j.tifs.2016.06.002.
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429, doi:10.3390/ANTIOX8100429.
- Gullón, B.; Gagaoua, M.; Barba, F.J.; Gullón, P.; Zhang, W.; Lorenzo, J.M. Seaweeds as promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends Food Sci. Technol. 2020, 100, 1–18, doi:10.1016/j.tifs.2020.03.039.
- Turgut, S.S.; Soyer, A.; Işıkçı, F. Effect of pomegranate peel extract on lipid and protein oxidation in beef meatballs during refrigerated storage. Meat Sci. 2016, 116, 126–132.
- Madane, P.; Das, A.K.; Pateiro, M.; Nanda, P.K.; Bandyopadhyay, S.; Jagtap, P.; Barba, F.J.; Shewalkar, A.; Maity, B.; Lorenzo, J.M. Drumstick (Moringa oleifera) flower as an antioxidant dietary fibre in chicken meat nuggets. Foods 2019, 8, 307, doi:10.3390/foods8080307.
- Banerjee, D.K.; Das, A.K.; Banerjee, R.; Pateiro, M.; Nanda, P.K.; Gadekar, Y.P.; Biswas, S.; McClements, D.J.; Lorenzo, J.M. Application of enoki mushroom (Flammulina velutipes) stem wastes as functional ingredients in processed meat. Foods 2020, 9, 432, doi:10.3390/foods9040432.
- de Carvalho, F.A.L.; Lorenzo, J.M.; Pateiro, M.; Bermúdez, R.; Purriños, L.; Trindade, M.A. Effect of guarana (Paullinia cupana) seed and pitanga (Eugenia uniflora) leaf extracts on lamb burgers with fat replacement by chia oil emulsion during shelf life storage at 2 °C. Food Res. Int. 2019, 125, 108554, doi:10.1016/j.foodres.2019.108554.
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166, doi:10.1016/j.foodres.2018.07.014.
- Lorenzo, J.M.; Vargas, F.C.; Strozzi, I.; Pateiro, M.; Furtado, M.M.; Sant’Ana, A.S.; Rocchetti, G.; Barba, F.J.; Dominguez, R.; Lucini, L.; et al. Influence of pitanga leaf extracts on lipid and protein oxidation of pork burger during shelf-life. Food Res. Int. 2018, 114, 47–54, doi:10.1016/j.foodres.2018.07.046.
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104.
- Bekhit, A.E.-D.A.; Hopkins, D.L.; Fahri, F.T.; Ponnampalam, E.N. Oxidative processes in muscle systems and fresh meat: Sources, markers, and remedies. Rev. Food Sci. Food Saf. 2013, 12, 565–597, doi:10.1111/1541-4337.12027.
- Bao, Y.; Ertbjerg, P. Effects of protein oxidation on the texture and water-holding of meat: A review. Rev. Food Sci. Nutr. 2019, 59, 3564–3578, doi:10.1080/10408398.2018.1498444.
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estevez, M. Protein oxidation in muscle foods: A review. Nutr. Food Res. 2011, 55, 83–95.
- Chauhan, P.; Pradhan, S.R.; Das, A.; Nanda, P.K.; Bandyopadhyay, S.; Das, A.K. Inhibition of lipid and protein oxidation in raw ground pork by Terminalia arjuna fruit extract during refrigerated storage. Asian-Australas. J. Anim. Sci. 2019, 32, 265–273, doi:10.5713/ajas.17.0882.
- Fernandez-Lopez, J.; Fernandez-Gines, J.M.; Aleson-Carbonell, L.; Sendra, E.; Sayas-Barbera, E.; Perez-Alvarez, J.A.; Fernández-López, J.; Fernández-Ginés, J.M.; Aleson-Carbonell, L.; Sendra, E.; et al. Application of functional citrus by-products to meat products. Trends Food Sci. Technol. 2004, 15, 176–185, doi:10.1016/j.tifs.2003.08.007.
- Hygreeva, D.; Pandey, M.C.; Radhakrishna, K. Potential applications of plant based derivatives as fat replacers, antioxidants and antimicrobials in fresh and processed meat products. Meat Sci. 2014, 98, 47–57, doi:10.1016/j.meatsci.2014.04.006.
- Madane, P.; Das, A.K.; Nanda, P.K.; Bandyopadhyay, S.; Jagtap, P.; Shewalkar, A.; Maity, B. Dragon fruit (Hylocereus undatus) peel as antioxidant dietary fibre on quality and lipid oxidation of chicken nuggets. Food Sci. Technol. 2020, 57, 1449–1461, doi:10.1007/s13197-019-04180-z.
- Basiri, S. Evaluation of antioxidant and antiradical properties of Pomegranate (Punica granatum) seed and defatted seed extracts. J. Food Sci. Technol. 2013, 52, 1117–1123, doi:10.1007/s13197-013-1102-z.
- Agregán, R.; Franco, D.; Carballo, J.; Tomasevic, I.; Barba, F.J.; Gómez, B.; Muchenje, V.; Lorenzo, J.M. Shelf life study of healthy pork liver pâté with added seaweed extracts from Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Food Res. Int. 2018, 112, 400–411.
- Smaoui, S.; Hlima, H.B.; Mtibaa, A.C.; Fourati, M.; Sellem, I.; Elhadef, K.; Ennouri, K.; Mellouli, L. Pomegranate peel as phenolic compounds source: Advanced analytical strategies and practical use in meat products. Meat Sci. 2019, 158, doi:10.1016/j.meatsci.2019.107914.
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Chem. Int. Ed. 2011, 50, 586–621.
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Valorization of Pomegranate Peels: A Biorefinery Approach. Waste Biomass Valorization 2017, 8, 1127–1137, doi:10.1007/s12649-016-9668-0.
- Gullón, P.; Astray, G.; Gullón, B.; Tomasevic, I.; Lorenzo, J.M. Pomegranate Peel as Suitable Source of High-Added Value Bioactives: Tailored Functionalized Meat Products. Molecules 2020, 25, 2859, doi:10.3390/molecules25122859.
- Morsy, M.K.; Mekawi, E.; Elsabagh, R. Impact of pomegranate peel nanoparticles on quality attributes of meatballs during refrigerated storage. LWT Food Sci. Technol. 2018, 89, 489–495, doi:10.1016/j.lwt.2017.11.022.
- Natalello, A.; Priolo, A.; Valenti, B.; Codini, M.; Mattioli, S.; Pauselli, M.; Puccio, M.; Lanza, M.; Stergiadis, S.; Luciano, G. Dietary pomegranate by-product improves oxidative stability of lamb meat. Meat Sci. 2020, 162, 108037, doi:10.1016/j.meatsci.2019.108037.
- Zago, G.R.; Gottardo, F.M.; Bilibio, D.; Freitas, C.P.; Bertol, C.D.; Dickel, E.L.; Dos Santos, L.R. Pomegranate (Punica granatum) peel lyophilized extract delays lipid oxidation in tuscan sausages. Cienc. Rural 2020, 50, doi:10.1590/0103-8478cr20190689.
- Zhuang, S.; Li, Y.; Jia, S.; Hong, H.; Liu, Y.; Luo, Y. Effects of pomegranate peel extract on quality and microbiota composition of bighead carp (Aristichthys nobilis) fillets during chilled storage. Food Microbiol. 2019, 82, 445–454, doi:10.1016/j.fm.2019.03.019.
- Yuan, G.; Lv, H.; Tang, W.; Zhang, X.; Sun, H. Effect of chitosan coating combined with pomegranate peel extract on the quality of Pacific white shrimp during iced storage. Food Control 2016, 59, 818–823, doi:10.1016/j.foodcont.2015.07.011.
- Tarkhasi, A. Effect of Edible Coating Containing Pomegranate Peel Extract on Quality and Shelf Life of Silver Carp (Hypophthalmichthys molitrix) Fillet during Refrigerated Storage. Food Ind. Microbiol. 2016, 2, doi:10.4172/2572-4134.1000112.
- Kandylis, P.; Kokkinomagoulos, E. Food applications and potential health benefits of pomegranate and its derivatives. Foods 2020, 9, 122, doi:10.3390/foods9020122.
- Kaderides, K.; Mourtzinos, I.; Goula, A.M. Stability of pomegranate peel polyphenols encapsulated in orange juice industry by-product and their incorporation in cookies. Food Chem. 2020, 310, doi:10.1016/j.foodchem.2019.125849.
- Zarezadeh Mehrizi, R.; Emam-Djomeh, Z.; Shahedi, M.; Keramat, J.; Rezaei, K.; Loni, E. Phenolic Compounds and Antioxidant Activity of Dried Peel of Iranian Pomegranate. Food Qual. Hazards Control 2017, 4, 103–108.
- De Oliveira, F.L.; Arruda, T.Y.; da Silva Lima, R.; Casarotti, S.N.; Morzelle, M.C. Pomegranate as a natural source of phenolic antioxidants. Food Bioact. 2020, 9, doi:10.31665/JFB.2020.9214.
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Antimicrobial potential of pomegranate peel: A review. J. Food Sci. Technol. 2019, 54, 959–965, doi:10.1111/ijfs.13964.
- Barati Boldaji, R.; Akhlaghi, M.; Sagheb, M.M.; Esmaeilinezhad, Z. Pomegranate juice improves cardiometabolic risk factors, biomarkers of oxidative stress and inflammation in hemodialysis patients: A randomized crossover trial. Sci. Food Agric. 2020, 100, 846–854, doi:10.1002/jsfa.10096.
- Parmar, H.S.; Kar, A. Medicinal Values of Fruit Peels from Citrus sinensis, Punica granatum, and Musa paradisiaca with Respect to Alterations in Tissue Lipid Peroxidation and Serum Concentration of Glucose, Insulin, and Thyroid Hormones. Med. Food 2008, 11, 376–381, doi:10.1089/jmf.2006.010.
- Hassan, N.A.; El-Halwagi, A.A.; Sayed, H.A. Phytochemicals, antioxidant and chemical properties of 32 pomegranate accessions growing in Egypt. World Appl. Sci. J. 2012, 16, 1065–1073.
- Rowayshed, G.; Salama, A.; Fadl, M.A.; Hamza, S.A.; Emad, A. Nutritional and Chemical Evaluation for Pomegranate (Punica granatum) Fruit Peel and Seeds Powders By Products. Middle East J. Appl. Sci. 2013, 3, 169–179.
- Venkitasamy, C.; Zhao, L.; Zhang, R.; Pan, Z. Pomegranate. In Integrated Processing Technologies for Food and Agricultural By-Products; Academic Press: Cambridge, MA, USA, 2019; pp. 181–216, ISBN 9780128141397.
- Bonesi, M.; Tundis, R.; Sicari, V.; Loizzo, M.R. The Juice of Pomegranate (Punica granatum): Recent Studies on Its Bioactivities. In Quality Control in the Beverage Industry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 459–489.
- Banihani, S.; Swedan, S.; Alguraan, Z. Pomegranate and type 2 diabetes. Res. 2013, 33, 341–348, doi:10.1016/j.nutres.2013.03.003.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26020467