Gemcitabine is an anticancer drug used to treat a wide range of solid tumors and is a first-line treatment for pancreatic cancer. The effect of gemcitabine is significantly weakened by its rapid plasma degradation. In addition, the systemic toxicity and drug resistance significantly reduce its chemotherapeutic efficacy. Up to now, many approaches have been made to improve the therapeutic index of gemcitabine. One of the recently developed approaches to improve conventional chemotherapy is based on the direct targeting of chemotherapeutics to cancer cells using the peptide-drug conjugates (PDCs).
- gemcitabine
- peptide-drug conjugates
- targeted drug delivery
- anticancer therapy
1. Introduction
Improving the therapeutic index of anticancer agents is an enormous challenge. In a time when the number of patients suffering from a cancer-related disease has been increasing each day and when conventional therapies gather a worrying number of deficits and drawbacks [1,2], new treatment options are required to relieve the symptoms and ultimately to eradicate the disease. Over the past decades, the development of new therapies that are more selective and less harmful to patients has been the target of many research groups [3,4]. However, these therapies still carry a risk of relapse and numerous side effects. The chemotherapeutic agents that target rapidly dividing cancer cells significantly damage healthy cells, especially those with rapid growth, such as bone marrow, gastrointestinal mucosa, and hair cells [5,6]. This causes the most common side effects of chemotherapy, such as myelosuppression (reduced blood cell production), inflammation of the lining of the gastrointestinal tract, and hair loss [6].
A modern approach to improve conventional chemotherapy is to focus on the direct targeting of chemotherapeutic agents to cancer cells [3,5]. Targeted drug delivery methods have been developed to improve drug efficacy and lower side effects by directing the drug to a specific cell type, enhance the tumoricidal effect, and reduce the peripheral toxicity of a specific drug [7]. Targeting decreases the side effects of therapeutic agents by delivering drugs to the intended destination [8]. To optimize the strategy of the anti-cancer agents targeting, one must ensure that the drug will not affect non-tumor-transformed cells and that the active substances will be transported in sufficient amounts to eliminate cancer cells and inhibit the tumor growth [8,9].
Designing the carriers of therapeutic substances is an approach that enables not only the improvement of pharmacokinetic properties and the biodistribution of traditional and innovative drugs, but also a reduction of their numerous side effects [9]. The binding of a drug to a carrier is often accompanied by a change in its mode of delivery, which is advantageous if it leads to the increased accumulation of the drug in the target tissue, e.g., in a cancer cell [5,8]. The biodistribution of such a conjugate is highly dependent on the properties of the carrier [10]. The synthesis of drug–peptide conjugates for the targeted delivery to a specific group of cells usually involves the conjugation of the drug with a targeting peptide via an appropriate linker, which could in turn facilitate the chemical or enzymatic release of the drug once the conjugate enters cancer cells via a receptor–mediated endocytosis mechanism [8,11]. The ester linkages, among several functional groups employed to connect the drug to the linker, have been widely utilized due to its possible release via an enzymatic (i.e., esterase–based) hydrolysis of the ester bond [12].
Several peptide-drug conjugates (PDCs) have been developed for different cancer types [13,14,15,16,17]. Some of these endeavors proceeded to clinical rating [16,17], while other provided crucial information about approaches that could enhance the stability of these molecules in circulation, thus improving their efficacy and reducing any associated toxicity [18].
Gemcitabine (2′,2′-difluorodeoxycytidine) (dFdC, Gem-used in the conjugate’s names)—a pyrimidine antimetabolite—is a prodrug with a demonstrated efficacy against a wide variety of cancers [19,20,21] and has been approved for use against colon, non-small cell lung, breast, pancreatic, bladder, and ovarian cancers [22,23]. Gemcitabine is transported into cells by nucleoside transporters: Human equilibrative (hENT1, hENT2) and concentrative nucleoside transporters (hCNT, hCNT2, and hCNT3) [24]. The drug molecule is then activated by deoxycytidine kinase (DCK)—a rate-limiting step for its pharmacological action—and phosphorylated to difluorodeoxycytidinemonophosphate (dFdCMP), which is further phosphorylated by the phosphate kinase enzyme into diphosphate (dFdCDP) and triphosphate (dFdCTP) forms. Both the dFdCDP and dFdCTP active forms show antitumor activity by the inhibition of the cellular DNA synthesis. dFdCTP incorporates into the DNA leading strand inhibiting DNA synthesis and subsequently leading to cell apoptosis. dFdCDP also has an indirect cytotoxic effect caused by the inhibition of ribonucleotide reductase. Finally, dFdC is rapidly metabolized into its inactive metabolite—2′,2′-difluoro-2′-deoxyuridine (dFdU)—by cytidine deaminase present in the blood, liver, and kidneys and is excreted through urine [25] (Figure 1).
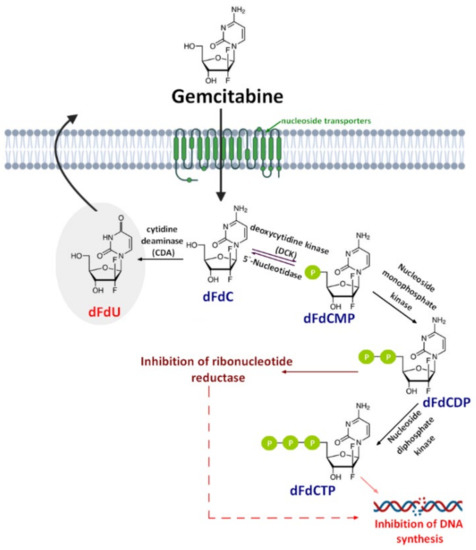
Despite the clinical successful application of gemcitabine, its short plasma half-life (9−13 min in human plasma) [26], poor diffusion into cells, and adverse toxicity—such as myelosuppression, the principal dose-limiting toxicities in gemcitabine cancer therapy—significantly reduce its chemotherapeutic potential [27,28]. This short half-life is the result of deamination of gemcitabine by cytidine deaminase which—as mentioned before—metabolize gemcitabine to the inactive dFdU [29]. Likewise, phosphorylated metabolites of gemcitabine are inactivated via the reduction by cellular 5′-nucleotidase (5′-NT) and then are rapidly removed from the body by the enzymatic conversion of gemcitabine [30,31]. Another important drawback associated with gemcitabine therapy is the drug resistance related to the nucleoside transporter deficiency, which is developed by some tumor cells after the initial tumor regression [32]. For this reason, many approaches have been made to improve the safety profile of gemcitabine and increase its chemotherapeutic index. These approaches include both chemical modifications either on the cytosine’s aniline or on the 5′-hydroxyl group of the 2,2′-difluoro-2′-deoxyribose moiety [33] and the novel drug delivery technology. Until now, various delivery strategies such as liposomes [34,35,36], nanoparticles [37,38], lipidic and nonlipidic derivatives [36], as well as poly(ethylene glycol) (PEG) and other polymeric drug conjugates [39,40] have been studied to prevent rapid plasma degradation and improve the selective delivery of gemcitabine to the tumor tissue. These approaches have been widely discussed earlier [25] and will not be discussed again herein.
An alternative strategy, which has recently attracted much more attention, was established by chemically conjugating hydrophilic drugs to cell-penetrating peptides (CPPs) [41,42]. CPPs are relatively short peptides, typically composed of less than 30 amino acid, that have been shown to be comparatively non-cytotoxic and capable of crossing the cell membrane. These peptides have been used to facilitate the transport of various therapeutic agents into cells, including plasmid DNA, siRNA, therapeutic proteins, viruses, imaging agents, and other various nanoparticles. The coupling of the anticancer drug to CPPs may result in numerous advantages, such as improved solubility, intracellular uptake, biodistribution, and pharmacokinetic profiles. Therefore, the CPP-based drug delivery system offers great potential for improving the intracellular delivery of therapeutic agents with poor permeability [42].
2. Gemcitabine Conjugated with Cell-Penetrating Peptides (CPPs)
In recent years, numerous natural and synthetic CPPs—such as TAT, transportan, penetratin peptides, and polyamino acids (e.g., poly-arginines)—were utilized for the intracellular delivery of anticancer agents [51,52]. Since all CPPs are able to efficiently pass through cell membranes while being non-cytotoxic and carry a wide variety of cargos inside cells, they are also used to form conjugates with gemcitabine.
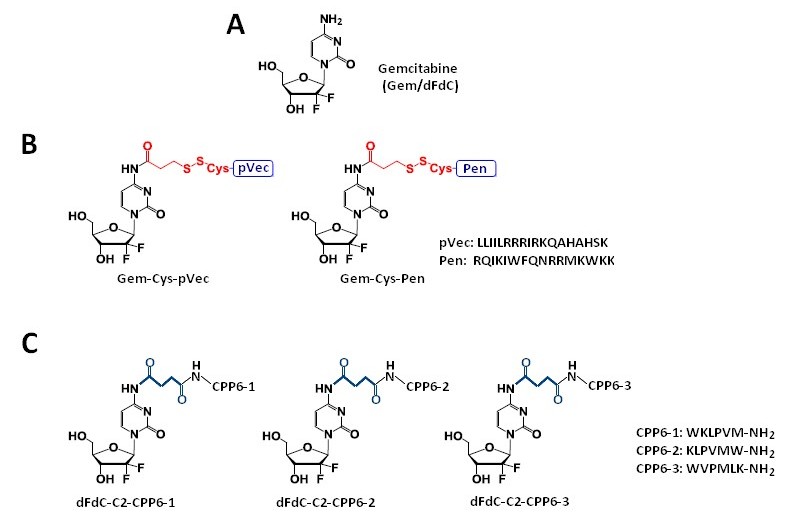
Vale et al. [41] synthesized two novel peptide–gemcitabine conjugates using two well-known CPP sequences—Penetratin (Pen, RQIKIWFQNRRMKWKK) [53] and pVEC (LLIILRRRIRKQAHAHSK) [54]. The authors connected these peptides with the aniline moiety of gemcitabine through the 3-sulfanylpropanoyl linker. They added an additional cysteine residue to the N-terminus of both CPPs to couple them with the linker, receiving Cys-Pen and Cys-pVec. A disulfide exchange reaction between Cys-modified CPPs and gemcitabine connected with the linker produced target conjugates (Figure 2B). The time-dependent kinetics of the gemcitabine release from hydrolysis of these new conjugates was studied in phosphate-buffered saline (PBS) at pH 7.4, 37 °C, and their biological activity was evaluated using three human cancer cell lines—MKN-28 (human gastric cancer), Caco-2 (heterogeneous human epithelial colorectal adenocarcinoma), and HT-29 (human colon adenocarcinoma). The results showed an increase in the anti-proliferative activity of gemcitabine in vitro upon conjugation with the CPP. Both CPP–gemcitabine conjugates (with the only exception of the Gem-Cys-pVEC conjugate against Caco-2 cells) worked substantially better than their components alone (either as a free drug or Cys-CPP) on the tested cell line (IC50 < 50 µM for conjugates vs. IC50 > 100 µM for gemcitabine and Cys-CPP). A semi-quantitative study of the degradation kinetics of both conjugates in PBS at pH 7.4, 37 °C, showed that gemcitabine is released upon the hydrolytic cleavage of the aromatic amide in Gem-Cys-Pen and Gem-Cys-pVec conjugates, with half-lives of approximately 9.6 days and 42 h, respectively. Based on the results mentioned above, the Vale’s group suggests that the remarkably higher stability of this conjugate may underlie its ability to make full use of its CPP moiety for enhanced internalization into the target cells, with a more controlled release of the parent drug over time [41].
In order to study the pharmacokinetics of gemcitabine-CPP conjugates and its constituents (gemcitabine and respective CPPs) and to establish a possible relation between the penetration potency of CPP and their physicochemical properties, Ferreira et al. [55] used the computational tool GastroPlus™—a powerful mechanistically-based simulation and modeling software for pharmaceutical research. Based on the simulations carried out in GastroPlus™, the authors stated that the conjugates’ bioavailability is ensured and the plasma concentration should reach therapeutic levels. The calculated AUC (area under the plasma concentration–time curve, μg-h/mL) for the conjugates was comparable to the AUC calculated for gemcitabine (~7.4404 and 7.4368, respectively). Yet, the estimated Cmax (maximum plasma concentration, in μg/mL) was higher for all the peptides and the analyzed conjugates (Cmax = 7.4403 μg/mL) compared with gemcitabine alone (Cmax = 5.9505 μg/mL). The Gem-Cys-pVEC conjugate binds less extensively to plasma proteins (>Fup, 42.89%). Bearing in mind that this conjugate showed the best in vitro bioactivity result for MKN-28 and HT-29 cells (IC50 = 20.68 µM and 45.20 µM, respectively; IC50 > 100 µM for gemcitabine) and released gemcitabine in PBS faster than the Gem-Cys-Pen conjugate (50% over 42 h versus 9.6 days for Gem-Cys-Pen) [41], the authors suggested that Gem-Cys-pVec conjugate has the most suitable profile for the drug delivery [55].
Continuing the previous studies, Vale’s group synthesized new series of gemcitabine-CPPs conjugates [32] containing three novel hexapeptides—CPP6-1, CPP6-2, and CPP6-3—which are the analogues of two peptides—KLPVM and VPMLK—derived from a family of CPP5 [56]. These peptides were reported to have a high ability to cross cell membranes. To improve their cell penetration capacity, additional tryptophan residues at their N- or/and C-termini were added and linked with gemcitabine using succinic anhydride, resulting in three novel gemcitabine-CPP6 conjugates (Figure 2C). Such a linker should improve the rate of drug delivery, as it protects the drug from cytidine deaminase (CDA) due to the conversion of its amino group to an amide moiety, comparatively unreactive under physiological conditions. To evaluate the in vitro cytotoxicity of the synthesized conjugates, the authors used human pancreatic adenocarcinoma (BxPC-3), human breast adenocarcinoma (MCF-7), and human prostate adenocarcinoma (PC-3) cancer cell lines. The results showed that two of three synthesized conjugates (dFdC-C2-CPP6-1 and dFdC-C2-CPP6-3) displayed a significantly higher cell growth inhibitory activity against PC-3 cells as compared with gemcitabine and CPPs constituent (after the conjugation with CPP6-1 and CPP6-3, gemcitabine IC50 decreased from 74 nM to 15 nM and 14 nM, respectively). Moreover, the three new conjugates of gemcitabine with CPP6 presented more potent cell growth inhibitory activity in MCF-7 and PC-3 cells (IC50 < 7 nM and IC50 ≤ 15 nM, respectively) than the reference drugs, tamoxifen (IC50 = 20 nM for MCF-7 and IC50 > 1000 nM for PC-3 cells) or metformin (IC50 = 9.9 nM for MCF-7 and IC50 = 189 nM for PC-3 cells) with the exception of the dFdC-C2-CPP6-2 conjugate for the PC-3 cell line (IC50 > 1000 nM). In addition, during this study the authors confirmed that in BxPC3 cells the dFdC-CPP6 conjugates are transported preferentially by hENT-1 transporter, and that once in the cytoplasm, dFdC-CPP6 conjugates may undergo sequential phosphorylations, disrupting DNA synthesis and causing apoptosis [32].
3. RGD Peptides-Gemcitabine Conjugates
Synthetic peptides containing the RGD sequence constitute one of the major classes of new pharmaceuticals with integrins as their primary therapeutic target. Such peptides act by binding to integrin receptors and destroying the mitochondria after cell penetration. This affects angiogenesis, thus disrupting the process of creating new blood vessels and inhibiting the tumor growth.
Unmodified RGD-peptide (Arg-Gly-Asp) binds specifically to αVβ3 and αVβ5 receptors, such as fibrinogen, fibronectin, vitronectin, plasminogen, thrombospondin, prothrombin, MMP-2, laminin, osteopontin, etc. which are excessively expressed on tumor cells and surfaces of vasculature, and is applied as an important component of a delivery system of various agents: Anticancer drugs, nanoparticles, imaging compounds, and virus vectors to tumor or angiogenic vessels [65,66,67,68,69]. The affinity of RGD peptides for their molecular targets may be affected by their conformation [70,71]. Apart from the direct interactions between peptide’s functional groups and their receptors, the conformational properties of the RGD motif can also be modified. The cyclization is a common modification, improving the binding features of RGD peptides and the rigidity of the molecule. Linear RGD peptides are strongly susceptible to the enzymatic degradation, but the introduction of conformational constrains caused by the cyclization averts this process, so cyclic peptides are more stable, more potent, and more specific. In cyclic peptides, the RGD-peptide sequence is surrounded by other amino acid residues to build a ring system. These modification offers the possibility to present the RGD sequence in a specific conformation for a selected integrin [70,71].
4. Conclusions
Drug targeting is crucial for effective cancer chemotherapy. Targeted delivery enhances chemotherapeutic effect and spares normal tissues from the toxic side effects of these powerful drugs. Up to now, many approaches have been made to improve the therapeutic index of gemcitabine. Peptide conjugates provide a valuable alternative to anti-cancer drugs used so far. A proper use of biological mechanisms of constituent peptide action can result in effective therapy for many diseases. Two groups of peptides were used to obtain gemcitabine conjugates—cell penetrating peptides and RGD peptides. Published results confirmed that peptides of both groups could be successfully applied to design new, efficient, and specific anti-cancer therapeutic agents, also in conjunction with the nanocarriers, such as nanoparticles, liposomes, or micelles. Enhanced pharmacological activity was achieved when components were non-covalently and covalently bond through the drug’s functional groups. The latter means that these groups could be subjected to the successful modification which usually is not the case for commercially available drugs. This again proves that gemcitabine is a very attractive leading structure to design gemcitabine conjugates with a potential to become new therapeutic tools for cancer therapy.
In conclusion, the use of gemcitabine peptide-based conjugates to treat cancer is a relatively new field and there are still many areas that need to be explored. However, these conjugates show great promise in the field of anticancer therapy because of their many benefits including no hematological or other toxicities, higher stability, and tissue specificity. One can expect that the successful transition from the laboratory to the clinic is only a matter of time and while the shift from the laboratory to the clinic is time consuming, and recent progress should promote this translation.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26020364
