Brain tumors are characterized by very high mortality and, despite the continuous research on new pharmacological interventions, little therapeutic progress has been made. One of the main obstacles to improve current treatments is represented by the impermeability of the blood vessels residing within nervous tissue as well as of the new vascular net generating from the tumor, commonly referred to as blood-brain barrier (BBB) and blood-brain tumor barrier (BBTB), respectively.
- brain cancer
- blood-brain barrier
- drug delivery
- FUS
- CED
- nanomedicine
1. Introduction
Tumors of the central nervous system (CNS) account for about 3% [1][2] of the worldwide diagnosed neoplastic diseases and represent one of the most frequent causes of solid tumor-related deaths in childhood [3]. More than 85% of the CNS tumors affect the brain, which is also a primary metastatic site for tumors originating in other organs including the bladder, breast, kidney, and lung [4]. Gliomas are the most common tumors of the brain, and they can originate from different cell phenotypes that constitute the glia (astrocytes, oligodendrocytes, microglia, ependymal cells). Further categorizations are based on cancer aggressiveness which is evaluated on a scale ranging from grade I to IV, with grade IV being the most malignant, challenging to treat and likely to reoccur. In this scenario, treatments vary from simple observation for grade I glioma (with 5–15 years median survival) to surgical resection in combination with radio and chemotherapy for grade IV glioma (with 9–12 months median survival). Resection is by far the most effective treatment at least in terms of mass tumor reduction, but it is limited by the structural complexity and the primary function of the brain. Tumor debulking is usually referred to as "maximal safe resection" [5], implying a high risk of cognitive loss following the surgical procedure and incomplete removal of the tumor. Surgical limitations contribute to the high incidence of brain cancer recurrence, usually detected within 2 cm from the primary tumor [6].
Glioblastoma multiforme (GBM) is the most common tumor of the brain in adults, representing about 50% of all diagnosed primary brain cancers and usually classified as a grade IV glioma [7]. GBM is characterized by cellular and molecular heterogeneity that makes the optimization of the pharmacological interventions very difficult. The Stupp protocol is the gold-standard treatment for GBM [8], and it consists of surgical resection, postoperative radiotherapy, and temozolomide (TMZ), often used in association with adjuvant therapies including carmustine and PCV (procarbazine, lomustine, and vincristine). Despite their significant cytostatic properties in vitro, many Food and Drug Administration approved chemotherapeutics have shown limited curative benefits in the clinic. In the case of brain tumors, the development of more effective treatments is hampered by the specialized barrier function that characterizes the blood vessels residing in the central nervous system and usually referred to as the blood-brain barrier (BBB). In its physiological function, the BBB thoroughly selects and controls the mass transport occurring in and out the brain, limiting the healthy (and tumor) tissue diffusion of the administered pharmaceuticals while increasing the therapeutic doses in the patients that do not respond to the treatments is rarely a viable option. Also, the new blood vessels originating from the neoplastic lesions and often referred to as blood-brain tumor barrier (BBTB) are significantly less permeable than the neovasculature of the tumors developing in other organs being that their development is driven by the nervous system microenvironment.
1.1. Anatomy of the BBB: Tight Junctions
The very first researcher that introduced the concept of BBB was Lena Stern [9], a pioneer in the neuroscience field that coined the term hematoencephalic barrier to describe the BBB. Other scientists worthy of mention for their contribution to the discovery of the BBB's functional and anatomical organization are Ehrlich, Lewandowsky, and Goldmann [10]. According to Sweeney et al. [11], the BBB is defined as "a continuous endothelial membrane within brain microvessels that has sealed cell-to-cell contacts and is sheathed by mural vascular cells and perivascular astrocyte end-feet." In the human, the BBB characterizes over 100 billion capillaries that cover a total length of around 400 miles and a surface area of 20 M2 [12]. BBB vessels control the exchange of circulating molecules, nutrients and gas between the blood and the nervous tissue. In its physiological function, the BBB protects the brain from larger particles, proteins and hydrophilic molecules including potential neurotoxins and bacteria. It is believed that only 2% of small molecules and 0% of the large molecules can cross the BBB. Theoretically, only highly hydrophobic molecules with a molecular mass not higher than 400–500 Da can diffuse through this barrier [13]. BBB properties are due to many factors including (but not limited to) highly selective cellular sorting mechanisms regulating the transcellular traffic and the expression of tight junctions (TJs) between adjacent endothelial cells, limiting the paracellular transport.
TJs are composed of different transmembrane proteins including (but not limited to) the family of claudins, occludin, and junctional adhesion molecules (JAM-A, -B, and -C) and they interact with the cell cytoskeleton through membrane-associated guanylate kinases called zonula occludens proteins (ZO-1, ZO-2, and ZO-3). It is believed that all these proteins have a pivotal role in determining BBB function and a specific work performed on claudin-5 demonstrated that inhibiting its expression increased BBB permeability for molecules as large as 800 kDa [14]. This demonstration highlights the fine regulation that stands at the basis of BBB permeability, suggesting that TJ targeting could be a viable strategy to increase it. The efficiency of these proteins in closing the gaps between endothelial cells can be experimentally evaluated in vitro by measuring transendothelial electric resistance (TEER) that determines the resistance associated with ionic transport via the transcellular and the paracellular route. In the case of proper BBB reconstruction, TEER needs to be significantly higher (at least above 900 Ω×cm2) than in other endothelial settings (2–20 Ω×cm2). This value is considered the cut-off for the permeability of IgG, considering this under physiological conditions, TEER values range from 1500 to 8000 Ω×cm2 [15][16]. However, these values can vary as a function of the animal origin and the quality of the endothelial cells (primary or immortalized cell lines) [16]. Usually, immortalized cell lines do not provide TEER values higher than 200 Ω×cm2 while endothelial cells derived from inducible pluripotent stem cells can provide TEER values higher than 1500 Ω×cm2. Recent discoveries highlighted the possibility that, despite their sealing action, these proteins could determine two distinct mechanisms of BBB crossing. The first is known as "charge pore pathway' in which the claudins form a molecular channel permeable only to small ions. The second is known as "size selective pathway" in which the passage to larger molecules occurs via a transient dissociation of TJ complexes [17]. A deeper understanding of these protein organizations could open new avenues of drug delivery as described later in the text.
1.2. Cellular and Enzymatic Elements of the Neurovascular Unit
The barrier function of the CNS endothelium is also determined by other cell phenotypes and biological structures including astrocytes, pericytes, microglia cells, neurons, and basement membranes which when taken with the endothelial cells, constitute what is commonly known as the neurovascular unit (Figure 1). Astrocytes are glial cells that interact with the endothelial cells through their polarized end-feet formations and control the BBB blood flow, development, and functions likely by enhancing the TJ expression in the mature BBB, even though they do not participate in its embryonic development [18][19]. In this context, some authors believe that astrocytes are not crucial for TJ expression, while others indicate that they can control TJ expression via Src-suppressed C-kinase substrates [20]. The modulation of BBB permeability occurs via secretion of important protein factors like the glial-derived neurotrophic factor, transforming growth factor-β1, basic fibroblast growth factor, interleukin 6, angiopoietin 1, retinoic acid, and Wnt [21][22]. Astrocytes also control the water exchange between intracellular, interstitial, vascular, and ventricular compartments by inducing the expression of the potassium channel kir4.1 and the water channel aquaporin-4. Pericytes have structural functions stabilizing the small BBB vessels and modulating the process of neovascularization and angiogenesis [23]. They are believed to significantly contribute to induce BBB gene expression as well as astrocyte end-feet polarization, even though more investigations are needed to reveal the complete spectrum of their activities in determining BBB and BBTB characteristics [24]. They control endothelial cell proliferation, survival, differentiation [18], and induce TJ mRNA expression in the embryonic formation of the BBB [25]. Microglia cells are the resident macrophages of the brain and contribute to the barrier function by modulating the innate immunity in the perivascular regions of the brain [22] and participating in the regulation of the expression of the TJ components [26]. Finally, neurons can induce the expression of TJ proteins like occludin and this phenomenon occurs synergistically with astrocytes [27]. BBB permeability also depends on enzymatic and immunological barriers limiting the molecular diffusion of blood solutes in the brain parenchyma. The endothelial cells composing the BBB express efflux transporters that are very efficient in transporting back to the luminal side the small hydrophobic molecules that crossed the BBB [28]. Efflux carriers are mostly adenosine triphosphate-binding cassette (ABC) transporters [27], and they are fundamental in clearing brain tissue from small lipophilic molecules. Between them, the P-glycoprotein (P-gp) and breast cancer resistance protein (ABCG2) were shown to have a significant role in the efflux of xenobiotics that penetrated the endothelial cell membrane, limiting the diffusion of chemotherapeutics in the brain parenchyma. P-gp is the most investigated pump, and its impact on brain transport was shown in knockout mice, where brain delivery increased up to 10–100 times [29]. This efflux pump is responsible for hampering the diffusion of many chemotherapeutics including doxorubicin (DOX), daunorubicin, vinblastine, vincristine, etoposide, and teniposide [30]. Also, together with the absence of endothelial fenestration, CNS endothelial cells showed a higher negative surface charge [31] and a lower transport rate through pinocytosis [32]. These parameters are highly considered for the designing and the development of more efficient delivery approaches (see later) since they constitute the physical and biological features of the BBB.
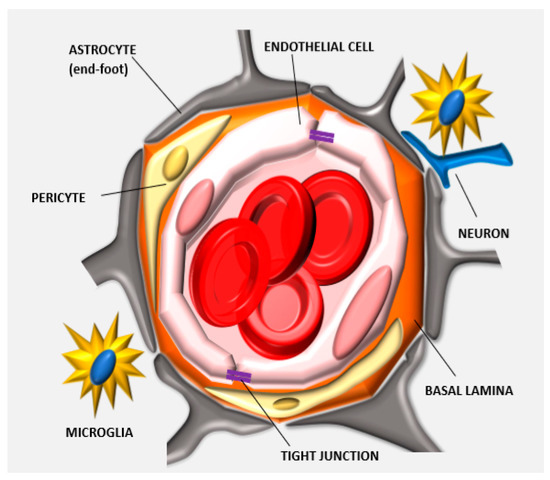
Figure 1. Anatomy of the neurovascular unit: the blood-brain barrier (BBB) structure is determined by different biological components that organize together in forming the neurovascular unit. Endothelial cells form the lumen of the capillary, interact with the basal lamina and the pericytes embedded in this matrix. The astrocytes, neurons, and microglia cells further support this cellular backbone. Other physical agents determining the barrier function of this specialized endothelium are the tight junctions (TJs) that are expressed between adjacent endothelial cells.
2. Models of BBB
One of the major obstacles in developing effective drug delivery across the BBB is the current lack of appropriate experimental in silico, in vitro and in vivo models allowing for cost-effective and high-throughput screening for different therapeutics. In silico models [33][34] of brain cancer are extensively developed for predicting tumor growth and infiltration in response to the treatments, while only a few cases are focused on predicting drug delivery in the brain neoplastic lesions [35][36]. The development of predictive computational models is critical in this field, also considering that mice have a brain structure extremely different from humans, counting for a 1:10 glial cell-to-neuron ratio versus a 1:5 ratio registered in humans [37]. Current in vitro and in vivo models are not reliable in mimicking and measuring BBB permeability respectively, but the research in this area is very active to discover new targets for favoring BBB accumulation as well as to understand the molecular dynamics that control TJ expression in the neurovascular unit.
2.1. Traditional In Vitro Models of BBB
Three important parameters need to be consistent in establishing in vitro models of BBB: (1) low permeability validated through high TEER values, (2) expression of specific BBB biomarkers (i.e., TJ components and specific transporters and enzymes) [38], and (3) evaluation of barrier integrity through specific size molecular markers (sodium fluorescein, lucifer yellow, fluorescein isothiocyanate (FITC)-inulin, FITC-dextrans, and FITC- bovine serum albumin) [39]. In vitro models vary from simple acellular systems to very complex, multi-phenotype cellular models. Acellular models usually consist of parallel artificial membrane permeability assays (PAMPA) [40] and are based on synthetic lipophilic membranes that can only partially reproduce the physical properties of the BBB in vivo. These membranes are used to predict the passive diffusion of molecules through the barrier as a function of their hydrophobic or hydrophilic character. Few attempts to isolate brain capillaries and test BBB properties ex vivo have been performed, but the complexity of the isolation protocols, low reproducibility, and the difficulties to flow the tested molecules in the lumen of the isolated blood vessels affect their ordinary use [40][41]. On the other hand, new advances in cell isolation allowed for reconstructing the BBB with endothelial cells isolated from the brain, even though non-endothelial surrogate cellular models (i.e., Caco-2, ECV304) [42], that can still express TJs, are used for research purposes [40]. Many attempts at reconstructing the neurovascular unit were performed by co-culturing the endothelium with astrocytes, C6 glioma cells, pericytes, mixed glial cells, and conditioned media. Two-dimensional (2D) in vitro models are generated by seeding the endothelial cells on the apical side of a porous membrane while interacting with another cell phenotype (i.e., astrocyte or pericyte) seeded on the other side of the membrane via cellular protrusions extended through the pores. A third cell phenotype can be included in the system by seeding it on the bottom of the well to generate a conditioned culture environment and allowing for investigating the direct effect of cancer cells on endothelial cells forming the BBB [43]. The system can be further refined by coating the porous membrane with proteins belonging to the basal lamina and by decreasing serum concentration to favor the movement of the TJs from the cytoplasm to the basolateral region of the cells [44]. The serum can contain protein factors (i.e., vascular endothelial growth factor) that increase the permeability of the reconstructed endothelium in vitro, while supplementing the media with hydrocortisone or Adenosine 3′,5′-cyclic monophosphate (cAMP) analogs can increase endothelial barrier function since this second messenger is involved in maintaining the ultrastructure conformation of the TJs [44].
2.2. D Models and In Vivo Methods to Evaluate BBB Permeability
Three-dimensional (3D) models are currently one of the most advanced technologies to reconstitute in vitro the BBB, and are constituted of different cell phenotypes including cancer cells, normal astrocytes, and endothelial cells. The cells can assembly in spheroid units supported by hydrogels, scaffolds, and adhesion molecules. The group of Pasqualini developed 3D spheroids (1 mm in diameter) through magnetic levitation, by seeding glioma cells on a hydrogel composed by gold, magnetic iron oxide nanoparticles, and filamentous bacteriophage targeting cell integrins to favor cell interactions [45]. They showed that the spheroids could resemble in vitro the protein expression of tumor biomarkers (N-cadherin) registered in vivo and that multiple cell phenotypes could be mixed in the same spheroid unit to investigate cell interaction, biology, and drug diffusion while providing effective implantable tumors. As it occurs in vivo, a necrotic core characterized the spheroids and, by modulating the external magnetic field, it was possible to control their size and shape. Also known as organ-on-chip, new advances in microfluidic devices were utilisied to better recapitulate the characteristics of the BBB tissue by combining geometrical, physical, and biological features of this tissue [46][47]. These tools can also be implemented with sensors providing real-time and continuous measurements of the changes occuring in BBB permeability under different conditions. These systems usually consist of polydimethylsiloxane that provides optimal integration with microscopy analysis and fine-tuned engineering via soft lithography on the microscale, which supports the organized culturing of cellular layers derived from the nervous tissue (i.e., endothelial cells, neurons, and astrocytes). In addition, they can be integrated with channels in which the media flows and supports the growth of endothelial cells to mimick the characteristics of primary tissue [48][49]. The different compartments allow for intercellular interactions to establish the critical cues of cellular communications for generating a functional BBB in vitro. In this scenario, the generation of refined 3D models can represent a breakthrough in the development of more advanced tools to investigate the biology of the neurovascular unit since they can: (1) include multiple interacting cell phenotypes and (2) evaluate BBB in flow conditions. However, to date, these systems are too complex to be ordinarily used worldwide and drug screening is still mostly performed in traditional transwell systems. For more information about these systems, we suggest the following reviews [16][50].
In vivo pharmacokinetic evaluation in the brain depends on different biological parameters including blood flow in the BBB, the density of influx and efflux transporters as well as the affinity of the drug for these transporters. The goal of these measurements is to quantify the product between the amount of therapeutic that crossed the BBB and the surface area of the BBB [51]. In vitro pharmacokinetics methods are not considered reliable because drug passive diffusion is generally over-estimated, while the active transport is frequently underestimated [52]. Different advanced techniques allow for calculating drug accumulation in the brain parenchyma like ex-vivo equilibrium dialysis performed on brain homogenates or slices or by using dialysis fibers directly implanted in vivo. This second method is generally preferred when possible because it allows for measuring drug concentration in the brain in the presence of normal blood flow. Also known as brain microdialysis, this method consists of implanting a small capillary in the brain parenchyma under continuous perfusion (Figure 2). The tip of the capillary is semipermeable and allows for collecting tissue fluids. However, the insertion of the capillary in the brain parenchyma could damage the BBB continuity with consequent leakage of blood fluid leading to an overestimation of the drug concentration. Overall there are three significant challenges in increasing brain drug delivery: (1) targeting the vasculature of the brain, (2) overcoming the BBB, and (3) favoring drug diffusion in the brain diseased tissue. In the next chapters, available information about current strategies for crossing the BBB will be described with a focus on their working mechanisms as well as the pros and cons of the different methods.
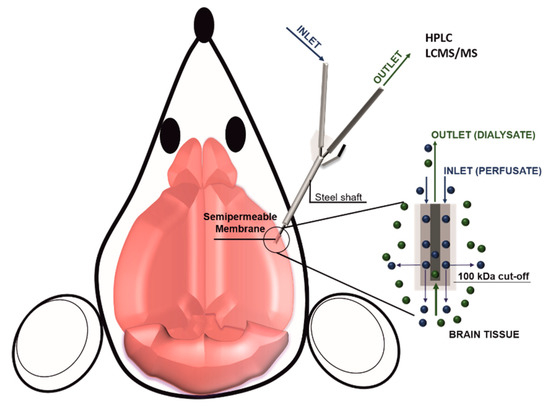
Figure 2. Scheme of brain microdialysis: A catheter is inserted in the brain tissue, while a controlled system (i.e., a syringe pump) injects in the brain a perfusate solution. At the end of the catheter is applied a semi-permeable membrane that allows for the injection of the perfusate, as well as for the collection of the dialysate composed by the perfusate and the brain tissue fluids. The collected dialysate can be eventually analyzed for its molecular content.
3. Breaching the BBB
Considering the importance of the brain and the physiological relevance of the BBB, barrier disruption by affecting TJ integrity and/or endothelial cell continuity has to be fine-tuned and reversible. These properties are fundamental because potential extravasation of circulating factors (i.e., albumin) can be very toxic for the neurons [53]. Traditional approaches to transiently affect BBB integrity are based on the injection of a hyperosmotic solution (usually consisting of a highly concentrated solution of mannitol [54]) just before the administration of the therapeutics. Hyperosmotic solutions can induce endothelial cell shrinking with a consequent increase in vascular leakage in the brain parenchyma. This approach was effective in increasing the overall survival of the patients (from 11 to 17 months), but it requires repeated hospitalization and is also considered very invasive (it needs patient sedation), unspecific, and accompanied by severe systemic toxicity, including neurological deficits, strokes, seizures, and new tumor-nodule formation [55]. Current clinical trials are devoted to optimizing the use of hyperosmotic solution based on mannitol [56] or NaCl [56] to increase chemotherapy and antibody delivery to the brain tumor and decrease intracranial pressure. Recently it was shown in rats that the osmotic disruption of the BBB (achieved via intracarotid injection of a 25% solution of mannitol) could be exploited to increase the delivery of hydrophobic siRNA, previously modified with phosphocholine (PC)-docosahexanoic acid. The increase in the hydrophobicity of this biological therapeutic was shown to enhance the retention of the siRNA in the brain without affecting its therapeutic action. The group of Chung developed a polymeric carrier of polydixylitol with high osmotic power that showed high efficiency in nucleic acid delivery in vitro and in vivo. More importantly, they showed that the osmotic BBB opening could induce caveolae-mediated transcytosis of the carriers while having a low toxicity profile [57].
4. Bypassing the BBB
There are essentially two extensively investigated pharmacological approaches that can be referred as to interstitial treatments for brain cancer: the application of biodegradable wafers and convection-enhanced delivery (CED), and both are designed to bypass the BBB. Generally, they are considered extremely invasive; however, both are already included in the clinical practice even though a lot of research is still dedicated to increasing their therapeutic benefits and their safety.
5. Negotiation of the BBB
New approaches of drug delivery aimed at negotiating the passage through the BBB have been proposed based on current knowledge of the transport mechanisms used by this specialized endothelium. Some of them exploit the physical properties of the BBB; others are based on the BBB biochemical receptor and transporter profile. To date, three main routes of BBB negotiation have been developed and referred to as adsorptive-mediated transcytosis (AMT), transporter-mediated transcytosis (TMT), and receptor-mediated transcytosis (RMT) (Figure 3). In this effort, the development of rationally designed nanocarrier surface modifications was shown to be useful to exploit these transport routes. Also, nanomedicine provided a mean to protect the encapsulated drug in the blood environment as well as to increase its bioavailability.
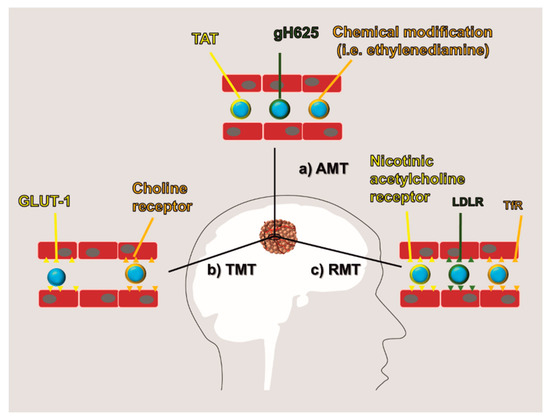
Figure 3. BBB negotiation: Current methods to negotiate BBB are obtained by modifying the therapeutic molecules or the carrier surface to increase their affinity for the BBB. They are generally referred to as: (a) adsorptive-mediated transcytosis (AMT) which is based on a positive surface charge of the therapeutics, (b) transporter-mediated transcytosis (TMT) which exploits the affinity of the therapeutics for endothelial transporters (i.e., GLUT1 and choline receptor), and (c) receptor-mediated transcytosis (RMT) which exploits the affinity of the therapeutics for endothelial receptors (i.e., nicotinic acetylcholine receptor, low-density lipoprotein receptor (LDLR), and transferrin receptor (TfR)).
6. Crossing Blood-Brain Tumor Barrier
Unlike the BBB, the BBTB has to be considered a pathological tissue since it is the product of the neoplastic lesion. Compared to regular BBB, BBTB is generally considered more permeable, even though as aforementioned, its barrier function (estimated cut-off of around 12 nm) [58] is significantly higher than what usually registered for the neo-vasculature generated from tumors in other organs. Even though the leaky behavior of BBTB can be appreciated also through regular MRI via brain edema detection, its dysfunction is not homogenous in the tumor tissue [28], and high functional variability was also appreciated between different patients. In the case of BBTB, the investigation of peculiar surface markers overexpressed in this tissue represent the best strategy to design carrier targeting, because it provides the opportunity to target the pathological tissue specifically. Despite the traditional targets described for BBB, BBTB can theoretically be targeted exploiting the typical surface biomarkers of growing blood vessels. For example, it was shown that targeting integrin ανβ3 through the cyclic RGD peptide applied on the surface of polymeric polylactic acid and polyethylenimine particles [59] increased the brain delivery of encapsulated nucleic acids and paclitaxel, respectively, when compared to non-functionalized carriers. Recent findings also demonstrated that brain drug delivery could benefit from strategies aimed at normalizing pathological vasculature like administration of the Ang2-binding and Tie2-activating antibody [60]. More importantly, the group of Koh demonstrated that this approach could eventually enhance brain drug delivery by decreasing the interstitial pressure while increasing blood vessels perfusion and tissue oxygen levels modulating immune cell infiltration [60]. The group of Moses analyzed commercial GBM cell lines and 70 tumor samples from patients affected by GBM and identified the integrin α2 (ITAG2) as a novel surface biomarker for this BBTB. This integrin is involved in cell migration and the surface functionalization of DOX-loaded liposomes (with an antibody specific for this protein) showed cytostatic effects in vitro and in vivo, highlighting the importance of more research in the discovery of novel endothelial surface biomarkers for the treatment of brain tumors [61]. Compared to the surrounding healthy tissue, a brain tumor is characterized by significant changes in cell metabolism tissue that can represent an important targeting cue. Albumin, for example, is normally excluded from the brain parenchyma by the presence of the BBB, but it was shown that neoplastic lesions can increase its uptake likely to exploit this circulating protein as a source of amino acids. The group of Huang demonstrated that brain cancer overexpressed secreted protein acidic and rich in cysteine (SPARC) and GP-60, increasing the albumin endothelial transcytosis and cancer uptake, respectively (Figure 4). To target these receptors, they generated albumin nanoparticles (100 nm) encapsulated with paclitaxel and fenretinide and modified their surface with a CPP to favor particle diffusion in the brain parenchyma [3]. Finally, it is worth mentioning that brain tumors can generate new blood vessels via vascular mimicry, a phenomenon that can occur as a drug resistance mechanism upon the use of anti-angiogenic adjuvant therapies [62]. Both in human and in pre-clinical models, it was shown that the presence of red blood cells within vessel walls lined up with cancer cells and basal lamina. These cells were positive to periodic acid-Schiff but negative to CD34 immune staining, excluding their endothelial nature. In this case, further investigation is necessary to understand the advantages of targeting vascular mimicry and potential therapeutic effects of this approach.
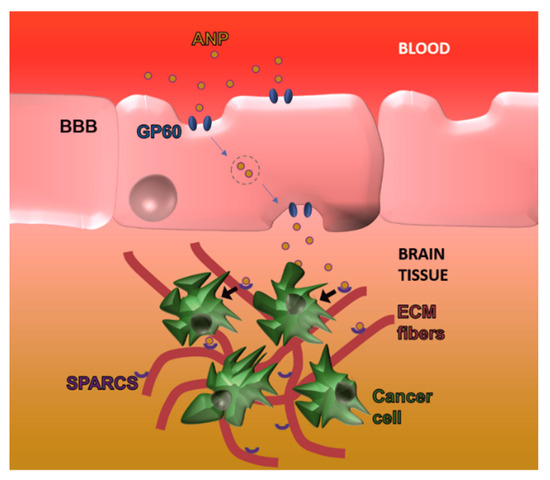
Figure 4. Exploiting tumor metabolic changes to overcome the BBB: Brain cancer lesions overexpress GP60 and secreted protein acidic and rich in cysteine (SPARC) that favor albumin nanoparticle trafficking over the BBB as well as cancer cell internalization in the abluminal side, respectively.
7. Cell and Gene Therapy
Approaches based on local delivery were used to inject healthy neural stem cells [63][64] to exploit their ability to infiltrate neoplastic lesions in the CNS. Genetically modified neural stem cells can be manipulated to generate and release cytotoxic molecules including prodrug-activating enzymes, apoptosis-inducing agents, antibodies [65], and oncolytic viruses [66]. The group of Portnow used neural stem cells modified for expressing cytosine deaminase to convert the prodrug 5-fluorocytosine (that can cross the BBB) to 5-fluorouracil. They directly injected the cells close to an established glioma or in the opposite hemisphere and they showed successful infiltration of the stem cells in the tumor parenchyma as well as higher cytostatic properties upon treatment with the prodrug [67]. Unfortunately, this procedure is affected by low efficiency in implanting viable cells. A way to avoid this issue is to seed the cells in vitro on a biocompatible scaffold (i.e., fibrin) and, like in the case of Gliadel, to insert the scaffold in the cavity obtained after brain tumor removal [68]. In this scenario, HEK 293 EBNA modified to release endostatin were encapsulated in an alginate scaffold prior to brain implantation, inhibiting in vivo GBM-induced angiogenesis process [69], while polymeric biodegradable scaffolds seeded with stem cells overexpressing secretable tumor necrosis factor apoptosis-inducing ligand were implanted to inhibit brain tumor growth [70]. Recent advances in biological drug delivery systems demonstrated that neutrophils could be exploited to overcome the BBB and increase drug delivery for brain cancer. The group Zhang loaded neutrophils in vitro with cationic liposomes and, after systemic administration, they infiltrated the neoplastic lesion guided by inflammatory cytokines and chemokines. The authors loaded the carriers with paclitaxel (which compared to other chemotherapeutics showed a minor impact on neutrophils biology) and exploited the cytokine gradient induced by the surgical removal of the tumor, exactly reproducing the clinical scenario [71]. Other biological agents used to treat brain cancer are adeno-associated viruses (AAV) since they are safe, effective, and one of the most promising methods to enhance gene delivery through the BBB [72][73]. The ability of different serotypes to effectively overcome the BBB is well known [74] even though the mechanism used to overcome BBB has still to be elucidated [75]. Engineering efforts have yielded several AAV variants that can efficiently transduce the CNS via systemic delivery in adult mice [76]. The group of Gao [74] tested nine different AAV vectors encoding green fluorescent protein (GFP) (injected into the superficial temporal vein of the mice) showing that they could increase GFP intensity in different brain compartments. Recently AAV targeted evolution technique revealed a novel recombinant AAV-PHP.B that transfers genes throughout the CNS with an efficiency that is at least 40-fold greater than that of the natural viruses [77]. Despite the optimistic perspectives regarding AAV-based delivery, some drawbacks need to be considered. The insert capacity of the vector is limited by 4 kb due to the AAV nature limiting some possible implementations of this method. In addition, the immune response to viruses can dramatically decrease the efficiency of gene transfer by systemic delivery. On the other hand, virus-induced expression of transgenes in the central nervous system can last for years [78], while the ectopic expression of the transgene can cause side effects. This limitation can be overridden by using cell type-specific promoters [76].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics11050245
References
- Mortazavi, S.; Mortazavi, S.; Paknahad, M. Cancers of the brain and CNS: Global patterns and trends in incidence. J. Biomed. Phys. Eng. 2018, 8, 151–152.
- Organization, W.H. Cancer Incidence and Mortality Worldwide: IARC; World Health Organization (WHO): Geneva, Switzerland, 2015.
- Lin, T.; Zhao, P.; Jiang, Y.; Tang, Y.; Jin, H.; Pan, Z.; He, H.; Yang, V.C.; Huang, Y. Blood–brain-barrier-penetrating albumin nanoparticles for biomimetic drug delivery via albumin-binding protein pathways for antiglioma therapy. ACS Nano 2016, 10, 9999–10012.
- Lin, X.; DeAngelis, L.M. Treatment of brain metastases. J. Clin. Oncol. 2015, 33, 3475–3484.
- Li, Y.M.; Suki, D.; Hess, K.; Sawaya, R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J. Neurosurg. 2016, 124, 977–988.
- Leuthardt, E.C.; Duan, C.; Kim, M.J.; Campian, J.L.; Kim, A.H.; Miller-Thomas, M.M.; Shimony, J.S.; Tran, D.D. Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS ONE 2016, 11, e0148613.
- Liu, H.-L.; Fan, C.-H.; Ting, C.-Y.; Yeh, C.-K. Combining microbubbles and ultrasound for drug delivery to brain tumors: Current progress and overview. Theranostics 2014, 4, 432–444.
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl. J. Med. 2005, 352, 987–996.
- Igual, M.M. Lina Stern (1878–1968) and the blood-brain barrier. A life between Geneva and Moscow. Neurosci. Hist. 2017, 5, 94–104.
- Shen, H.H. Core Concept: Circumventing the blood–brain barrier. Proc. Natl. Acad. Sci. USA 2017, 114, 11261–11263.
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150.
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201.
- McDannold, N.; Zhang, Y.; Power, C.; Arvanitis, C.D.; Vykhodtseva, N.; Livingstone, M. Ultrasound-mediated blood-brain barrier disruption for targeted drug delivery in the central nervous system. In Proceedings of Micro-and Nanotechnology Sensors, Systems, and Applications VII; Baltimore Convention Center Baltimore: Baltimore, MD, USA, 2015; p. 94670H.
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5–deficient mice. J. Cell Biol. 2003, 161, 653–660.
- Bicker, J.; Alves, G.; Fortuna, A.; Falcão, A. Blood–brain barrier models and their relevance for a successful development of CNS drug delivery systems: A review. Eur. J. Pharm. Biopharm. 2014, 87, 409–432.
- DeStefano, J.G.; Jamieson, J.J.; Linville, R.M.; Searson, P.C. Benchmarking in vitro tissue-engineered blood–brain barrier models. Fluid. Barriers CNS 2018, 15, 32.
- Zeniya, S.; Kuwahara, H.; Daizo, K.; Watari, A.; Kondoh, M.; Yoshida-Tanaka, K.; Kaburagi, H.; Asada, K.; Nagata, T.; Nagahama, M. Angubindin-1 opens the blood–brain barrier in vivo for delivery of antisense oligonucleotide to the central nervous system. J. Control. Release 2018, 283, 126–134.
- Persidsky, Y.; Ramirez, S.H.; Haorah, J.; Kanmogne, G.D. Blood–brain barrier: Structural components and function under physiologic and pathologic conditions. J. Neuroimmu. Pharmacol. 2006, 1, 223–236.
- Daneman, R.; Prat, A. The blood–brain barrier. Cold Spring Harbor Perspect. Biol. 2015, 7, a020412.
- Lee, S.-W.; Kim, W.J.; Choi, Y.K.; Song, H.S.; Son, M.J.; Gelman, I.H.; Kim, Y.-J.; Kim, K.-W. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat. Med. 2003, 9, 900–906.
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41.
- Correale, J.; Villa, A. Cellular elements of the blood-brain barrier. Neurochem. Res. 2009, 34, 2067.
- Trost, A.; Lange, S.; Schroedl, F.; Bruckner, D.; Motloch, K.A.; Bogner, B.; Kaser-Eichberger, A.; Strohmaier, C.; Runge, C.; Aigner, L. Brain and retinal pericytes: Origin, function and role. Front. Cell. Neurosci. 2016, 10, 20.
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K. Pericytes regulate the blood–brain barrier. Nature 2010, 468, 557–561.
- Hori, S.; Ohtsuki, S.; Hosoya, K.i.; Nakashima, E.; Terasaki, T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J. Neurochem. 2004, 89, 503–513.
- Zenker, D.; Begley, D.; Bratzke, H.; Rübsamen-Waigmann, H.; von Briesen, H. Human blood-derived macrophages enhance barrier function of cultured primary bovine and human brain capillary endothelial cells. J. Physiol. 2003, 551, 1023–1032.
- Schiera, G.; Bono, E.; Raffa, M.P.; Gallo, A.; Pitarresi, G.L.; Di Liegro, I.; Savettieri, G. Synergistic effects of neurons and astrocytes on the differentiation of brain capillary endothelial cells in culture. J. Cell. Mol. Med. 2003, 7, 165–170.
- Oberoi, R.K.; Parrish, K.E.; Sio, T.T.; Mittapalli, R.K.; Elmquist, W.F.; Sarkaria, J.N. Strategies to improve delivery of anticancer drugs across the blood–brain barrier to treat glioblastoma. Neuro-oncology 2015, 18, 27–36.
- Chen, Z.; Shi, T.; Zhang, L.; Zhu, P.; Deng, M.; Huang, C.; Hu, T.; Jiang, L.; Li, J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016, 370, 153–164.
- Cho, H.; Lee, H.-Y.; Han, M.; Choi, J.-r.; Ahn, S.; Lee, T.; Chang, Y.; Park, J. Localized down-regulation of P-glycoprotein by focused ultrasound and microbubbles induced blood-brain barrier disruption in rat brain. Sci. Rep. 2016, 6, 31201.
- Ribeiro, M.; Domingues, M.; Freire, J.; Santos, N.; Castanho, M. Translocating the blood-brain barrier using electrostatics. Front. Cell. Neurosci. 2012, 6, 44.
- McGowan, J.W.; Bidwell, G.L., III; Vig, P.J. Challenges and new strategies for therapeutic peptide delivery to the CNS. Ther. Deliv. 2015, 6, 841–853.
- Liu, H.; Dong, K.; Zhang, W.; Summerfield, S.G.; Terstappen, G.C. Prediction of brain: Blood unbound concentration ratios in CNS drug discovery employing in silico and in vitro model systems. Drug Discov. Today 2018, 23, 1357–1372.
- Sharma, B.; Luhach, K.; Kulkarni, G. In vitro and in vivo models of BBB to evaluate brain targeting drug delivery. In Brain Targeted Drug Delivery System; Elsevier: Amsterdam, Netherlands, 2019; pp. 53–101.
- Arvanitis, C.D.; Askoxylakis, V.; Guo, Y.; Datta, M.; Kloepper, J.; Ferraro, G.B.; Bernabeu, M.O.; Fukumura, D.; McDannold, N.; Jain, R.K. Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood–tumor barrier disruption. Proc. Natl. Acad. Sci. USA 2018, 115, E8717–E8726.
- Boujelben, A.; Watson, M.; McDougall, S.; Yen, Y.-F.; Gerstner, E.R.; Catana, C.; Deisboeck, T.; Batchelor, T.T.; Boas, D.; Rosen, B. Multimodality imaging and mathematical modelling of drug delivery to glioblastomas. Interface Focus 2016, 6, 20160039.
- Begley, D.J. Brain superhighways. Sci. Transl. Med. 2012, 4, 147fs129.
- Kuo, Y.-C.; Lu, C.-H. Effect of human astrocytes on the characteristics of human brain-microvascular endothelial cells in the blood–brain barrier. Colloids Surf. B Biointerfaces 2011, 86, 225–231.
- Patabendige, A.; Skinner, R.A.; Abbott, N.J. Establishment of a simplified in vitro porcine blood–brain barrier model with high transendothelial electrical resistance. Brain Res. 2013, 1521, 1–15.
- Mensch, J.; Jaroskova, L.; Sanderson, W.; Melis, A.; Mackie, C.; Verreck, G.; Brewster, M.E.; Augustijns, P. Application of PAMPA-models to predict BBB permeability including efflux ratio, plasma protein binding and physicochemical parameters. Int. J. Pharm. 2010, 395, 182–197.
- Burek, M.; Salvador, E.; Förster, C.Y. Tissue-based in vitro and ex vivo models for blood–brain barrier permeability studies. In Concepts and Models for Drug Permeability Studies; Elsevier: Amsterdam, Netherlands, 2016; pp. 343–356.
- Kaisar, M.A.; Abhyankar, V.V.; Cucullo, L. In Vitro BBB Models: Working with Static Platforms and Microfluidic Systems. In Blood-Brain Barrier; Springer: New York, NY, USA, 2019; pp. 55–70.
- Mendes, B.; Marques, C.; Carvalho, I.; Costa, P.; Martins, S.; Ferreira, D.; Sarmento, B. Influence of glioma cells on a new co-culture in vitro blood–brain barrier model for characterization and validation of permeability. Int. J. Pharm. 2015, 490, 94–101.
- Brown, R.C.; Morris, A.P.; O’Neil, R.G. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res. 2007, 1130, 17–30.
- Souza, G.R.; Molina, J.R.; Raphael, R.M.; Ozawa, M.G.; Stark, D.J.; Levin, C.S.; Bronk, L.F.; Ananta, J.S.; Mandelin, J.; Georgescu, M.-M. Three-dimensional tissue culture based on magnetic cell levitation. Nat. Nanotechnol. 2010, 5, 291–296.
- Brown, J.A.; Codreanu, S.G.; Shi, M.; Sherrod, S.D.; Markov, D.A.; Neely, M.D.; Britt, C.M.; Hoilett, O.S.; Reiserer, R.S.; Samson, P.C. Metabolic consequences of inflammatory disruption of the blood-brain barrier in an organ-on-chip model of the human neurovascular unit. J. Neuroinflamm. 2016, 13, 306.
- Maoz, B.M.; Herland, A.; FitzGerald, E.A.; Grevesse, T.; Vidoudez, C.; Pacheco, A.R.; Sheehy, S.P.; Park, T.-E.; Dauth, S.; Mannix, R. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat. Biotechnol. 2018, 36, 865.
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129.
- Adriani, G.; Ma, D.; Pavesi, A.; Goh, E.; Kamm, R. Modeling the blood-brain barrier in a 3D triple co-culture microfluidic system. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 338–341.
- Van Der Helm, M.W.; Van Der Meer, A.D.; Eijkel, J.C.; van den Berg, A.; Segerink, L.I. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 2016, 4, e1142493.
- Bickel, U. How to measure drug transport across the blood-brain barrier. NeuroRx 2005, 2, 15–26.
- Pardridge, W.M. CSF, blood-brain barrier, and brain drug delivery. Expert Opin. Drug Deliv. 2016, 13, 963–975.
- Liu, Z.; Liu, J.; Wang, S.; Liu, S.; Zhao, Y. Neuronal uptake of serum albumin is associated with neuron damage during the development of epilepsy. Exp. Ther. Med. 2016, 12, 695–701.
- Choi, C.; Kim, H.M.; Shon, J.; Park, J.; Kim, H.-T.; Oh, S.-H.; Kim, N.K.; Kim, O.J. Additional increased effects of mannitol-temozolomide combined treatment on blood-brain barrier permeability. Biochem. Biophys. Res. Commun. 2018, 497, 769–775.
- Rodriguez, A.; Tatter, S.; Debinski, W. Neurosurgical techniques for disruption of the blood–brain barrier for glioblastoma treatment. Pharmaceutics 2015, 7, 175–187.
- NIH. Clinicaltrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=brain+tumor&term=mannitol&cntry=&state=&city=&dist= (accessed on 20 April 2019).
- Garg, P.; Pandey, S.; Seonwoo, H.; Yeom, S.; Choung, Y.-H.; Cho, C.-S.; Choung, P.-H.; Chung, J.H. Hyperosmotic polydixylitol for crossing the blood brain barrier and efficient nucleic acid delivery. Chem. Commun. 2015, 51, 3645–3648.
- Wei, X.; Chen, X.; Ying, M.; Lu, W. Brain tumor-targeted drug delivery strategies. Acta Pharm. Sin. B 2014, 4, 193–201.
- Huang, Y.; Liu, W.; Gao, F.; Fang, X.; Chen, Y. c (RGDyK)-decorated Pluronic micelles for enhanced doxorubicin and paclitaxel delivery to brain glioma. Int. J. Nanomed. 2016, 11, 1629–1641.
- Park, J.-S.; Kim, I.-K.; Han, S.; Park, I.; Kim, C.; Bae, J.; Oh, S.J.; Lee, S.; Kim, J.H.; Woo, D.-C. Normalization of tumor vessels by Tie2 activation and Ang2 inhibition enhances drug delivery and produces a favorable tumor microenvironment. Cancer Cell 2016, 30, 953–967.
- Guo, P.; Moses-Gardner, A.; Huang, J.; Smith, E.R.; Moses, M.A. ITGA2 as a potential nanotherapeutic target for glioblastoma. Sci. Rep. 2019, 9, 6195.
- Angara, K.; Rashid, M.H.; Shankar, A.; Ara, R.; Iskander, A.; Borin, T.F.; Jain, M.; Achyut, B.R.; Arbab, A.S. Vascular mimicry in glioblastoma following anti-angiogenic and anti-20-HETE therapies. Histol. Histopathol. 2017, 32, 917–928.
- Aboody, K.; Najbauer, J.; Danks, M. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008, 15, 739–752.
- Frank, R.T.; Najbauer, J.; Aboody, K.S. Concise review: Stem cells as an emerging platform for antibody therapy of cancer. Stem Cells 2010, 28, 2084–2087.
- Frank, R.T.; Edmiston, M.; Kendall, S.E.; Najbauer, J.; Cheung, C.-W.; Kassa, T.; Metz, M.Z.; Kim, S.U.; Glackin, C.A.; Wu, A.M. Neural stem cells as a novel platform for tumor-specific delivery of therapeutic antibodies. PLoS ONE 2009, 4, e8314.
- Ahmed, A.U.; Thaci, B.; Alexiades, N.G.; Han, Y.; Qian, S.; Liu, F.; Balyasnikova, I.V.; Ulasov, I.Y.; Aboody, K.S.; Lesniak, M.S. Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma. Mol. Ther. 2011, 19, 1714–1726.
- Aboody, K.S.; Najbauer, J.; Metz, M.Z.; D’apuzzo, M.; Gutova, M.; Annala, A.J.; Synold, T.W.; Couture, L.A.; Blanchard, S.; Moats, R.A. Neural stem cell–mediated enzyme/prodrug therapy for glioma: Preclinical studies. Sci. Transl. Med. 2013, 5, ra159–ra184.
- Sheets, K.T.; Bagó, J.R.; Hingtgen, S.D. Delivery of Cytotoxic Mesenchymal Stem Cells with Biodegradable Scaffolds for Treatment of Postoperative Brain Cancer. In Targeted Drug Delivery; Springer: New York, NY, USA, 2018; pp. 49–58.
- Read, T.-A.; Farhadi, M.; Bjerkvig, R.; Olsen, B.R.; Rokstad, A.M.; Huszthy, P.C.; Vajkoczy, P. Intravital microscopy reveals novel antivascular and antitumor effects of endostatin delivered locally by alginate-encapsulated cells. Cancer Res. 2001, 61, 6830–6837.
- Kauer, T.M.; Figueiredo, J.-L.; Hingtgen, S.; Shah, K. Encapsulated therapeutic stem cells implanted in the tumor resection cavity induce cell death in gliomas. Nat. Neurosci. 2012, 15, 197–204.
- Xue, J.; Zhao, Z.; Zhang, L.; Xue, L.; Shen, S.; Wen, Y.; Wei, Z.; Wang, L.; Kong, L.; Sun, H. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 2017, 12, 692–700.
- Challis, R.C.; Kumar, S.R.; Chan, K.Y.; Challis, C.; Beadle, K.; Jang, M.J.; Kim, H.M.; Rajendran, P.S.; Tompkins, J.D.; Shivkumar, K. Systemic AAV vectors for widespread and targeted gene delivery in rodents. Nat. Protoc. 2019, 14, 379–414.
- Kotterman, M.A.; Schaffer, D.V. Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 2014, 15, 445–451.
- Zhang, H.; Yang, B.; Mu, X.; Ahmed, S.S.; Su, Q.; He, R.; Wang, H.; Mueller, C.; Sena-Esteves, M.; Brown, R. Several rAAV vectors efficiently cross the blood–brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Mol. Ther. 2011, 19, 1440–1448.
- Choudhury, S.R.; Hudry, E.; Maguire, C.A.; Sena-Esteves, M.; Breakefield, X.O.; Grandi, P. Viral vectors for therapy of neurologic diseases. Neuropharmacology 2017, 120, 63–80.
- Bedbrook, C.N.; Deverman, B.E.; Gradinaru, V. Viral strategies for targeting the central and peripheral nervous systems. Annu. Rev. Neurosci. 2018, 41, 323–348.
- Deverman, B.E.; Pravdo, P.L.; Simpson, B.P.; Kumar, S.R.; Chan, K.Y.; Banerjee, A.; Wu, W.-L.; Yang, B.; Huber, N.; Pasca, S.P. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 2016, 34, 204–209.
- Montgomery, K.L.; Iyer, S.M.; Christensen, A.J.; Deisseroth, K.; Delp, S.L. Beyond the brain: Optogenetic control in the spinal cord and peripheral nervous system. Sci. Transl. Med. 2016, 8, rv335–rv337.
