Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nursing
Metabolic syndrome (MetS), a cluster of abdominal obesity, hyperglycemia, hypertriglyceridemia, low HDL (high density lipoprotein)-cholesterol, and hypertension, is known to be a strong risk factor for type 2 diabetes and is considered one of the most important preventable risk factors for cardiovascular diseases, whose prevalence is increasing.
- metabolic syndrome
- quality of life
- healthy lifestyle
- health education
- exercise
- diet therapy
1. Introduction
Cardiovascular diseases (CVD) are the leading causes of death in the world, accounting for a combined 15.2 million deaths in 2016. These diseases have remained the leading causes of death globally in the last 15 years [1]. Metabolic syndrome (MetS), a cluster of abdominal obesity, hyperglycemia, hypertriglyceridemia, low HDL (high density lipoprotein)-cholesterol, and hypertension, is known to be a strong risk factor for type 2 diabetes and is considered one of the most important preventable risk factors for CVD [2,3,4], whose prevalence is increasing. It is estimated that 39.9 ± 0.7% of the Asian population, 29.2 ± 0.7% of the European population, and 34.3 ± 0.8% of the adult US population suffers from MetS [5,6,7].
The main treatment for MetS prevention is a change in lifestyle through a multifactor approach based on education, regular physical exercise, and a healthy diet. An increasing number of studies support the idea that these changes in lifestyle were efficient in achieving the proposed goals for the treatment of MetS [6]. However, most physicians treat each component of MetS separately, prioritizing the treatment of those components that are easily amenable with drug treatment, given that it is easier to prescribe a drug to lower blood pressure, blood glucose, or triglycerides than to initiate a long-term strategy to change a person’s lifestyle [8].
It is important to highlight that MetS may lead to alterations in self-perceived well-being, since MetS has been linked to a decrease in health-related quality of life (HRQoL) [9,10]. Interestingly, these alterations in HRQoL may encourage the development of lifestyle changes more than the comorbidities associated with MetS itself [11]. Nevertheless, the relationship between MetS and HRQoL is complicated, with previous studies suggesting that the relationship may vary for the different components, physical and mental, of HRQoL. Moreover, some results are inconsistent, especially in relation to the mental component [12,13,14]. There are several validated questionnaires that measure the HRQoL, but the SF-36 (The Short Form-36 Health Survey) is the most widely used as an accurate way to measure self-perceived HRQoL. This questionnaire consists of 36 items that assess eight dimensions or scales, and these dimensions are grouped into two health components: the physical component summary (PCS) and the mental component summary (MCS). Each item received a numerical score that was encoded, summed up, and put on a scale from 0 to 100. The higher the score, the better the quality of life in the analyzed field [15,16,17].
In addition, there are also few studies evaluating the influence of lifestyle interventions on HRQoL in individuals with MetS [18,19,20,21,22,23,24,25,26,27,28]. There has been one systematic review of published studies which suggests that MetS is associated with reduced physical and mental HRQoL in cross-sectional studies, but, as of yet, no meta-analyses [29]. The systematic review of randomized clinical trials (RCTs) [29] reported improvements in metabolic parameters and HRQoL through lifestyle-based interventions. However, there was disagreement about which dimensions were most affected.
Therefore, the aim of this meta-analysis is to assess the effects of lifestyle interventions on physical and mental HRQoL through health education on nutrition, physical activity, and healthy habits in adults with metabolic syndrome.
2. Description of Studies
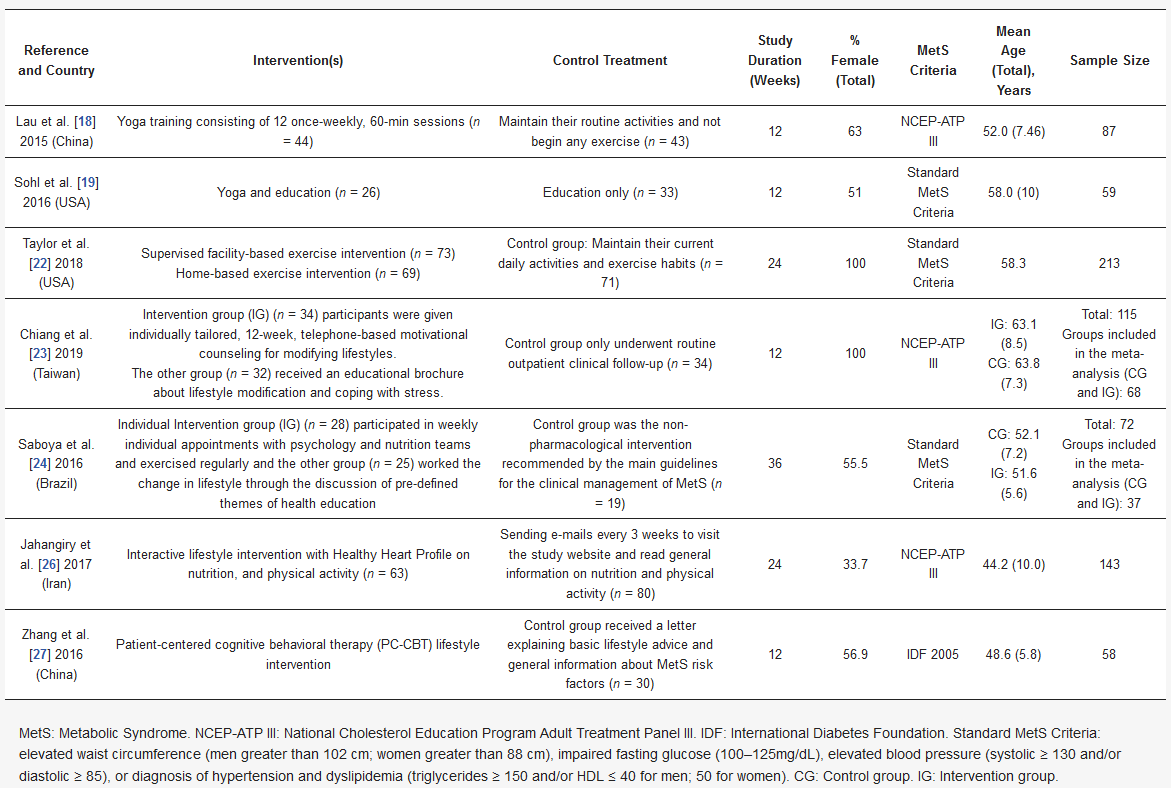
The electronic search identified 321 publications (Figure 2). We used three different databases that, when combined, found 89 duplicates, which were then excluded. The titles of the 232 remaining publications were reviewed, and 174 articles were found not to fulfill the inclusion criteria and were excluded. There were 58 relevant records, of which the abstract and full text were reviewed. Of these, 21 were excluded after reading the abstract because they did not fulfill the inclusion criteria. The full text articles reviewed included 22 non-randomized design studies and four that were of other designs and characteristics; these were excluded. Among the remaining 11 publications, four did not use the SF-36 questionnaire, two of which also had a high risk of bias (Table 1) [18,19,20,21,22,23,24,25,26,27,28]. Table 2 provides a detailed overview of the seven RCTs selected for meta-analysis, including information about the intervention(s), control treatment, study duration (weeks), percentage female, MetS criteria, mean age, and sample size. The earliest published study included was from 2015. Four of the studies were conducted in Asia (57.1%) [18,23,26,27], two in North America (28.6%) [19,22] and one in South America (14.3%) [24]. The study duration lasted from 12 to 36 weeks and the average participant was 54.7 years old. In addition, two studies were conducted on only male participants [22,23], while the remainder included both sexes. The total number of participants was 637, and all participants had at least two criteria for MetS. Interventions were described as lifestyle and exercise intervention, and control treatment was described as general information about nutrition and physical activity, or maintaining their current daily activities and exercise habits. For those studies with three comparison groups, their characteristics were evaluated to choose the intervention group to be included in the meta-analysis. In the case of Chiang et al. and Saboya et al. [23,24], the intervention group selected was the one that was subjected to an individualized and proactive intervention. For the Taylor et al. study, the two intervention groups were analyzed as one, according to the authors’ criteria [22]. All studies reported results for both the physical and mental health components of SF-36.
Table 2. Summary of 7 randomized controlled trials included in meta-analysis.
3. Study Quality and Risk of Bias
Table 1 shows the quality measures of the randomized controlled trials. All studies included in the meta-analysis were at low risk of bias and reported using random sequence generation. Three of the studies had no information about allocation concealment, three provided this information, and one did not carry it out. Blinding was performed in three studies and none of the studies included reported incomplete and selective outcome data. In addition, publication bias was assessed using a funnel plot for physical and mental health scores (Figure 3). Egger’s test provided statistical evidence of funnel plot asymmetry in the physical health scores, suggesting the presence of a significant publication bias (p < 0.001). For the mental health scores, no significant publication bias was detected (p = 0.1078).
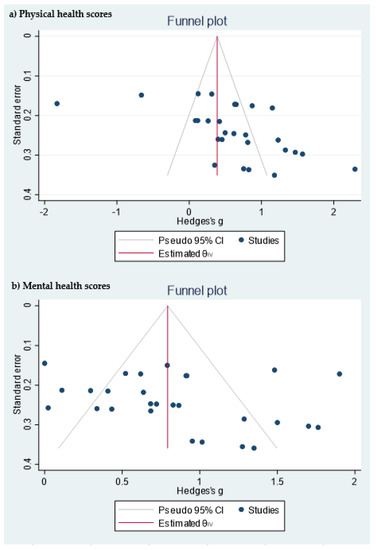
Figure 3. Funnel plot of the (a) physical health scores and (b) mental health scores.
4. Physical Health-Related Quality of Life
All seven studies that included the SF-36 questionnaire reported scores for the four physical dimensions in a format that permitted quantitative meta-analysis. In all dimensions, we found significant improvements in the intervention group with respect to the control group (Figure 4). The dimension with the greatest Hedges’ g difference between the two groups was General Health (GH): active intervention (n = 331) compared with the control group (n = 306) (Hedges’ g 0.76 points, 95% CI = 0.41–1.12, p < 0.001, I2 = 77.82%, 95% CI = 53.63–89.27).
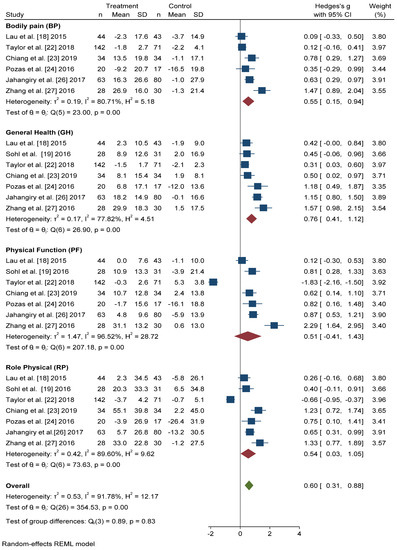
Figure 4. Forest plot of the physical health scores. Blue navy square centered at the point estimate of the effect size, with a horizontal line extending on either side of the square, representing the 95% confidence interval of the point stimate. Red diamond represents a confidence interval for each dimensions and green diamond represents a confidence a confidence interval for the overall effect size. REML: Restricted maximum-likelihood.
The differences in Hedges’ g for Bodily Pain (BP) between the intervention group and the control group was 0.55 points, 95% CI = 0.15–0.94, I2 = 80.71%, 95% CI = 52.06–90.14. We also found significant improvements in the scores of Physical Function (PF) (Hedges’ g 0.51 points, 95% CI = −0.41–1.43, p < 0.001, I2 = 96.52%, 95% CI = 95.65–98.07) and Role Physical (RP) (Hedges’ g 0.54 points, 95% CI = −0.03–1.05, p < 0.001, I2 = 89.60%, 95% CI = 85.77–95.33) in the active intervention compared with the control group.
In overall change scores, significant improvement was found in subjects receiving the active intervention compared to the group that received general lifestyle information (Hedges’ g 0.61 points, 95% CI = 0.31–0.91). Substantial heterogeneity was present (I2 = 92.04%, 95% CI = 90.46–94.36).
5. Mental Health-Related Quality of Life
Figure 5 shows that the scores obtained in the Mental Health (MH) and Social Function (SF) dimensions were similar, and significant improvement was found with active intervention (n = 331) compared with the control group (n = 306) (Hedges’ g 0.71 points, 95% CI = 0.37–1.05, p < 0.001, I2 = 75.86%, 95% CI = 34.57–86.35 and Hedges’ g 0.76 points, 95% CI = 0.37–1.15, p < 0.001, I2 = 81.84%, 95% CI = 67.59–91.64, respectively).
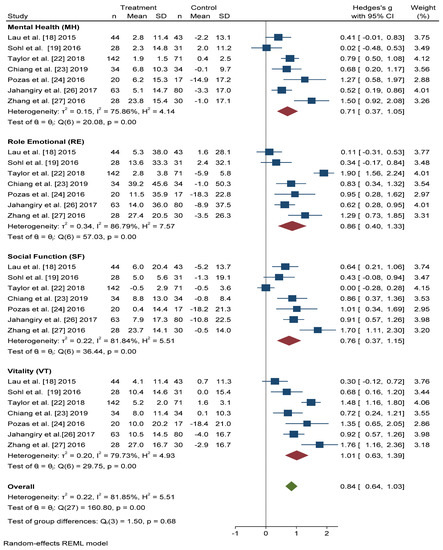
Figure 5. Forest plots of the mental health scores. Blue navy square centered at the point estimate of the effect size, with a horizontal line extending on either side of the square, representing the 95% confidence interval of the point stimate. Red diamond represents a confidence interval for each dimensions and green diamond represents a confidence a confidence interval for the overall effect size. REML: Restricted maximum-likelihood.
For the Role Emotional (RE) domain, better results were also obtained in the intervention group than in the control group (Hedges’ g 0.86 points, 95% CI = 0.40–1.33, I2 = 86.79%, 95% CI = 80.84–94.22). The Vitality (VT) domain score improved, to a greater extent, in actively treated subjects compared with the control group (Hedges’ g 1.01, 95% CI = 0.63–1.39, I2 = 79.73%, 95% CI = 58.83–90.12).
In overall change scores, significant improvement was found in subjects receiving the active intervention compared to the group that received general lifestyle information (Hedges’ g 0.84 points, 95% CI = 0.64–1.03, I2 = 81.85%, 95% CI = 76.68–87.91).
4. Discussion
In this meta-analysis of RCTs, we identified seven randomized trials that examined the impact of a lifestyle intervention on HRQoL in individuals with MetS. Significant improvements in HRQoL were found in those subjects who received the active lifestyle intervention compared with the usual care group. These improvements occur in all scores in the dimensions that make up the physical and mental component summary.
There is a great deal of scientific evidence regarding the association between a healthy diet and physical activity in improving HRQoL in subjects with MetS [34,35], and now our study suggests that active lifestyle interventions in individuals with MetS were determinants in HRQoL. A previous systematic review was published in 2016 on studies examining the association between MetS and HRQoL [29], but to our knowledge, this is the first meta-analysis of the effect of lifestyle interventions on HRQoL in adults with MetS. Systematic review results show that MetS is significantly associated with worsening HRQoL and, furthermore, intervention studies for lifestyle modification in subjects with MetS demonstrate significant results in improving HRQoL after the intervention. However, some of these studies show association only in women, or only with depression or higher body mass index (BMI).
Published scientific literature has reported that intensive lifestyle intervention improves anthropometric and metabolic parameters in individuals with MetS, and results in a significant association between overweight and obesity and impairment in the physical domain of HRQoL [36,37,38]. However, results of data on the impact of MetS on HRQoL are inconsistent. Given the importance of BMI in analyzing the relationship between HRQoL and MetS, it would be interesting to consider whether BMI would be a parameter to include in the measurement of the effectiveness of interventions. Only two of the RCTs included in this meta-analysis took into account the relationship between BMI and the HRQoL [22,24]. Usually, the physical spheres most affected were PF and GH, which could explain why a significant improvement was found in our study in the GH after the active intervention [13,39,40].
There was a significant improvement in all four domains of the mental health field, which was surprising, given that in previous studies the improvement in the mental spheres is confusing. In a cross-sectional study published by Vetter et al. [9], participants with MetS that were enrolled in a primary care-based weight reduction trial concluded that MetS was not associated with HRQoL as assessed using two generic instruments. However, a cross-sectional study published by Roohazfza et al. [41] in an Isfahan Cohort Study concluded the association of MetS with depression, anxiety, psychological distress, and quality of life. These differences may be due to the study design and the questionnaires used; our study shows that in the RCTs analyzed, after an intensive intervention in individuals with MetS, scores on the mental dimensions of the SF-36 questionnaire were better in the intervention group than in the control group.
This study had some limitations that need to be highlighted. First was the duration of the intervention, because the longest study was 36 weeks and did not show whether the improvement in quality of life was maintained over time. Second was the low number of RCTs published on these subjects and the small sample size included. We tried to overcome this limitation by performing the meta-analysis, which includes more than 600 individuals, and has therefore become one of our strengths. Third, the study of the differences between women and men is essential when discussing HRQoL and MetS. However, the characteristics of the studies did not allow the analyses to be carried out by sex. Furthermore, given the small sample size and the small number of selected articles, it would be possible to increase the variability. Finally, some analysis presents high heterogeneity, and due to the scarcity of articles it is not possible to unravel the differences between the different types of intervention and their duration. We believe that the results of the meta-analysis are promising, however, more and better research is needed in order to decrease the heterogeneity of the analyses.
An additional strength of this study was the inclusion of only published studies reporting SF-36 scores. SF-36 has been translated and validated in more than 50 countries and is the most commonly used measure. However, this was the first meta-analysis published on the impact of a lifestyle intervention on HRQoL in subjects with MetS, and it seems clear that there is a publication bias and significant heterogeneity in the evaluation of physical spheres. As such, the promising results detected in this meta-analysis are extremely important but they should be interpreted with caution. It is necessary to carry out further research in this field.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph18030887
This entry is offline, you can click here to edit this entry!
