Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
Metformin (MF), a first-line drug to treat type 2 diabetes mellitus (T2DM), alone and in combination with other drugs, shows positive effect on steroidogenesis and spermatogenesis in men with T2DM and metabolic syndrome, thus MF treatment indicates prospective use for improvement of male reproductive functions and fertility in these disorders. The entry is focused on use of MF therapy for restoration of male reproductive functions in metabolic and endocrine disorders.
- Metformin and Male reproduction
1. Introduction
The signaling pathways of metformin (MF) in cells are still not fully understood, they seem to be dependent on species and cell type, as well as doses and routes of administration, along with metabolic and hormonal status of subjects [22,24,25,26,27].
The molecule of MF, a small hydrophilic cation, is transported from the extracellular space to the cytoplasm of the target cell through organic cation transporters-1 and -2 (OCT1, OCT2), multidrug and toxin extrusion transporters (MATE), and ATM (ataxia telangiectasia mutated) transporter, and OCT1 and OCT2 are considered as the main functional units of MF transmembrane transport [28]. The ultimate intracellular target for MF is the 5′-adenosine monophosphate-activated protein kinase (AMPK), the key energy sensor of the cell, although MF does not interact directly with the enzyme [22,30,31,32]. In pathological conditions, like type 2 diabetes mellitus (T2DM) and metabolic syndrome (MetS), the activity of AMPK is reduced. MF’s action increases the activity of AMPK, and consequently normalizes the energy metabolism of the target cell. The AMPK consists of a catalytic α-subunit and the regulatory β- and γ-subunits that form a functionally active αβγ-heterotrimeric complex, and is widely distributed in all subcellular compartments (cytoplasmic, lysosomal, mitochondrial, and nuclear). AMPK is activated by increasing levels of AMP, a positive allosteric regulator of the enzyme [31,33,34]. The interaction of AMP with the adenine nucleotides-binding sites located in the γ subunit leads to stabilization of the αβγ heterotrimeric complex and enables phosphorylation of the α-subunit by liver kinase B1 (LKB1), which leads to the increase in AMPK activity [31,32,35] (Figure). Activating phosphorylation of AMPK may be also mediated by Ca2+-calmodulin-dependent protein kinase kinase 2 (CaMKK2) [36,37] and transforming growth factor β activated kinase-1 (TAK1) [38,39,40], but LKB1 is most important for AMPK activation [31,34,41,42,43]. Allosteric binding of AMP and ADP to γ-subunit of AMPK increases the ability of LKB1 and CaMKK2 to phosphorylate AMPK α-subunit at the Thr172 [44,45,46]. In the lysosomes, the “non-canonical” pathway of LKB1-mediated AMPK activation is carried out through dissociation of fructose 1,6-bisphosphate from aldolase. At the lysosomal surface, free aldolase promotes the formation of a multiprotein complex, including the vacuolar H+-ATPase and the scaffold protein AXIN, and this complex ensures the effective binding between AMPK and LKB1, thereby activating AMPK [47,48]. A negative regulator of AMPK is the protein phosphatase 2C (PP2C), which dephosphorylates and inactivates the α-subunit of AMPK, causing the dissociation of the αβγ-heterotrimeric complex. Elevated levels of AMP lead to an inhibition of PP2C activity, which allows AMPK to remain stable in the active Thr172-phosphorylated state [49,50].
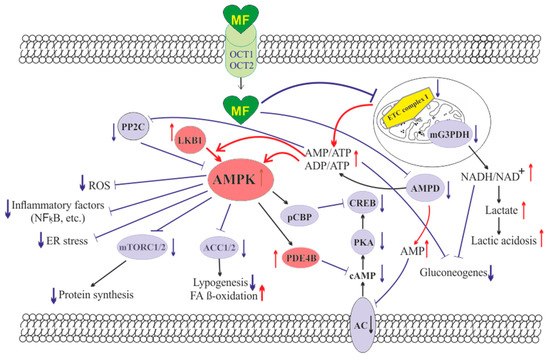
Figure. The cellular mechanisms of metformin action which are carried out by activation of the AMP-activated protein kinase and inhibition of the mitochondrial electron transport chain complex I. Abbreviations: AC, adenylyl cyclase; ACC1/2, acetyl-CoA carboxylases 1 and 2; AMPD, AMP deaminase; AMPK, the heterotrimeric AMP-activated protein kinase consisting of the α1/2 (the target for activation phosphorylation at the Thr172), β1/2 and γ1/2/3 subunits; CREB, cAMP-activated transcription factor (cAMP response element-binding protein); ETC complex I, the mitochondrial NADH-dehydrogenase complex, the first complex of the respiratory electron transport chain; FA, fatty acids; LKB1, liver kinase B1; mG3PDH, mitochondrial glycerol-3-phosphate dehydrogenase; mTORC2, the mTOR complex 2; NFκB, nuclear factor κB; OCT1/2, the organic cations transporters 1 and 2; pCBP, the Ser436-phosphorylated form of CREB-binding protein with acetyltransferase activity, a co-activator of the factor CREB; PDE4B, cAMP-specific 3′,5′-cyclic phosphodiesterase 4B; PKA, cAMP-dependent protein kinase; PP2C, protein phosphatase 2C; ROS, reactive oxygen species.
MF penetrates into the mitochondria through intracellular space and accumulates in them. While in the mitochondria, MF inhibits the mitochondrial ETC complex I, which leads to decrease in ATP production and increase in the [AMP]i/[ATP]i and [ADP]i/[ATP]i ratios [51,52,53,54]. Moreover, MF decreases the activity of the enzyme AMP-deaminase (AMPD), which converts AMP to inosine monophosphate, inducing the accumulation of AMP within the cell [55]. The MF-induced increase in the intracellular AMP level leads to the activation of AMPK as described above [41,56]. The MF effect on AMPK activity is observed at drug concentrations below 80 μM, which are achieved with oral administration of therapeutic doses of MF [57]. The MF-induced activation of AMPK results in the stimulation of energy-producing catabolic pathways that mediate the increased glucose uptake by cells, the increased expression and activity of the membrane glucose transporters, the activated metabolic processes such as glycolysis and oxidative phosphorylation, and the normalization of mitochondrial biogenesis [20,24,58,59]. The MF-induced AMPK stimulation leads to phosphorylation of types 1 and 2 acetyl-CoA carboxylases (ACC1 and ACC2), inducing an inhibition of lipogenesis and stimulation of the β-oxidation of free fatty acids [60,61,62] (Figure). The ultimate results of this metabolic cascade is the decrease of T2DM- and MetS-produced dyslipidemia, and the normalization of lipid metabolism. In addition, the AMPK activation induces a plethora of cellular events, including regulation of autophagy and apoptotic processes, a decrease in the activity of inflammatory factors, including nuclear factor κB (NF-κB) and interleukin 1β, an inhibition of the ROS production, a decrease in the ER stress, as well as a decrease in insulin/IGF-1-induced activation of the mTORC1/2 complexes and a decrease in the protein synthesis [60,62,63,64,65,66].
The MF is a functional antagonist of cAMP-dependent signaling cascades, which are stimulated by hormones, glucagon in particular, through the Gs protein-coupled receptors and the membrane-bound forms of adenylyl cyclase (AC) [67,68]. The stimulation of AC results in an increase in the intracellular cAMP level and the activation of the protein kinase A (PKA) and the cAMP-activated transcription factor CREB (cAMP response element-binding protein). The MF-induced activation of AMPK promotes phosphorylation and activation of cAMP-specific 3′,5′-cyclic phosphodiesterase 4B (PDE4B), thereby reducing the intracellular level of cAMP [68]. Moreover, MF causes an increase in the intracellular level of AMP, a negative regulator of the catalytic site of AC, which leads to inhibition of AC activity and a decrease in cAMP production. An increase in the level of AMP can be the result of both inhibition of the mitochondrial ETC complex I, and suppression of the activity of AMP deaminase [55,69] (Figure). A decrease in the activity of cAMP-dependent pathways in the liver, like activation of AMPK, leads to the inhibition of glucose synthesis in hepatocytes. Furthermore, MF-induced AMPK activation induces the protein kinase ι/λ-mediated phosphorylation of cyclic AMP response element binding (CREB)-binding protein (CBP or CREBBP) at the Ser436, which leads to the inability of the phospho-CBP to form a functionally active complex with the factor CREB and thereby inhibits the cAMP-dependent gene transcription [70].
Along with AMPK-dependent, there are also AMPK-independent pathways of MF action on the intracellular effector systems and gene expression. High-dose MF inhibits the activity of the mitochondrial glycerol-3-phosphate dehydrogenase (mG3PDH) [71]. The inhibition of mG3PDH leads to an increase in NADH levels and decreases NAD+ levels, and this causes a deficiency in NAD+, which is involved in the conversion of lactate to pyruvate (Figure). Since a decrease in mG3PDH activity inhibits the conversion of lactate to glucose, the result of impaired gluconeogenesis in hepatocytes is an accumulation of lactate, which can cause lactic acidosis in the conditions of high-dose MF treatment [71,72]. Another target of MF is the enzyme H3K27me3-demethylase KDM6A/UTX, which is responsible for the transcriptional activity of a large number of genes [73].
The antidiabetic effects of MF may be due to the changes in the gut microbiota, due to stimulation of the growth of bacteria that produce short-chain fatty acids [74]. By modulating the composition of the microbiota in rodents with T2DM and MetS, MF reduces the levels of bacterial lipopolysaccharides in the blood [75], and activates AMPK-dependent pathways in the mucosal layer of the intestine, reducing glucose absorption [76].
The most important mechanism of action of MF on target cells is the enhancement of the insulin signaling pathways and the decrease in insulin resistance (IR). This may be due to inhibition of hyperactivated nuclear factor κB (NF-κB), a transcription factor that provokes the development of IR, as well as a decrease in the expression of the phosphatase and tensin homolog (PTEN), which dephosphorylates phosphatidylinositol-3,4,5-triphosphate and thereby prevents insulin-induced stimulation of Akt kinase, a key effector component in the 3-phosphoinositide signaling pathway. The inhibitory effect of MF on the activity of NF-κB-dependent signaling pathways is carried out mainly through the stimulation of AMPK [25,77,78]. Since NF-κB plays a key role in inflammatory reactions, its inhibition by MF promotes the weakening of inflammation and increases the cell survival, and these effects of MF are prevented by AMPK inhibitors [25,79,80].
2. Metformin and the Male Reproduction
2.1. Effects Metformin on the Male Reproduction in Metabolic Disorders
Men with type 2 diabetes mellitus (T2DM), metabolic syndrome (MetS) and severe obesity have hypogonadotropic hypogonadism and other reproductive dysfunctions that are usually associated with impaired spermatogenesis and steroidogenesis [411,412,413,414,415,416,417,418,419,420,421,422]. The main factors that negatively affect the spermatogenesis and steroidogenesis in these metabolic disorders are dyslipidemia, IR, hormonal dysregulation in the Leydig and Sertoli cells, imbalance of cytokines in the testes, and an increase in the inflammation and the apoptotic and oxidative processes in testicular and germ cells [414,418,423,424,425].
A recent meta-analysis of 11 clinical studies involving 1731 men with MetS showed a significant decrease in total sperm count, sperm concentration, sperm survival, and the number of sperm with normal morphology and progressive motility, as well as an increase in sperm DNA fragmentation and functional changes in the mitochondrial energy and biogenesis [426]. Along with an androgen deficiency, the blood levels of follicle-stimulating hormone (FSH) and inhibin B were significantly decreased in men with MetS, while the blood levels of luteinizing hormone (LH), estrogen, and anti-Müllerian hormone (AMH) were changed to a small extent [426].
In contrast to patients with MetS, in T2DM, the results of studies of the sperm parameters, androgenic status and gonadotropins levels differed greatly, which was due to the age heterogeneity, differences in the severity and duration of T2DM, and the features of the etiology and pathogenesis of this disease [417]. Some authors showed the pathological changes in spermatogenesis, including a decrease in the number, motility and survival of spermatozoa and an increase in their defective forms and DNA fragmentation [418,427]. Meanwhile, a meta-analysis carried out by Greek scientists in 2016 indicates a decrease in seminal volume and the number of motile spermatozoa in men with T2DM, while the total number of sperm and their normal forms did not change significantly [417]. It can be assumed that the arterial hypertension, typical for MetS and T2DM, negatively affects morphology of the seminiferous tubules and spermatogenesis. Using the rat models, it was shown that arterial hypertension, interfering with normal microcirculation of blood in the testes, led to the impaired sperm maturation [428].
Since MF improves the lipid and carbohydrate metabolism, increases insulin sensitivity, prevents overproduction of reactive oxygen species and pro-inflammatory factors in men with MetS and T2DM, the use of MF therapy should thereby have a restorative effect on reproductive functions in men with these metabolic disorders [94,429]. Indeed, there are the experimental and clinical evidences of a positive effect of MF on the male reproductive dysfunctions in MetS and T2DM, but the mechanisms and the factors influencing the effectiveness of the restorative MF action are still poorly understood [94,430,431]. In addition, a number of authors did not confirm the improving effect of MF on male reproduction or even showed its negative effect on androgenic status in men with severe obesity and T2DM [432,433,434].
In addition to the MF effects in the testes, which is well documented (see the Section 2.2 and Section 2.3 for details), the targets of MF can also be hypothalamic gonadotropin-releasing hormone (GnRH)-expressing neurons and pituitary gonadotrophs, the other links of the male hypothalamic-pituitary-gonadal (HPG) axis. However, there are currently no data on the direct influence of MF on GnRH-expressing neurons and gonadotrophs in men. At the same time, it is known that MF, when acting on the primary cultures of pituitary cells, is able to influence the expression of LH and FSH, thereby controlling the synthesis of gonadotropins in them [435].
2.2. The Clinical Studies of the Metformin Efficacy to Treat Reproductive Dysfunctions in Men
The first reliable evidence of the efficacy of MF therapy in improvement of the reproductive functions in men with MetS was presented in 2010 [436]. Four-month therapy of 35 men with MF (1700 mg/day), which was combined with a balanced normocaloric diet and physical activity, led to a decrease in insulin levels and the homeostasis model assessment of insulin resistance (HOMA-IR) index and an increase in the blood levels of total and free testosterone. Additively, in men with hypogonadotropic hypogonadism, the MF-induced normalization of FSH levels was shown [436]. These data provided evidence of a restorative effect of MF on the hormonal status of the HPG axis in men with MetS and androgen deficiency.
Subsequently, Giuseppe Morgante and colleagues studied the restorative effect of MF (850 mg/day for the first week, 1700 mg/day for the second week, and 2550 mg/day for the rest six-month period) on spermatogenic function and hormonal indices of the HPG axis in 45 men with MetS who had the impaired spermatogenesis, including oligospermia, teratozoospermia, and asthenozoospermia. MF increased the number of spermatozoa, improved their motility and morphology and, thereby, partially restored fertility [430]. In MF-treated men, the blood testosterone and LH levels were significantly increased, while the levels of estradiol and sex hormone-binding globulin (SHBG) were decreased, which led to an increase in the testosterone/estradiol ratio and an improvement of androgenic status. The normalization of LH levels induced by MF indicates the restoration of the hypothalamic mechanisms responsible for pulsatile LH release [430]. Since an improvement in spermatogenesis and the hormonal status of the HPG axis was associated with a 43% decrease in the HOMA-IR index, this confirms the leading role of IR and glucose intolerance in development of spermatogenesis dysfunctions and androgen deficiency in men with MetS.
One clinical study investigated the effect of a combined three-month treatment of men with impaired glucose tolerance or with T2DM using MF at a daily dose of 2000 mg and clomiphene citrate (CC) at a low dose (25 mg) on their androgenic status [434]. The CC is characterized by the ability to significantly improve hormonal parameters in men with hypogonadotropic hypogonadism, primarily by normalizing the secretion of gonadotropins [437,438]. The combined therapy with MF plus CC led to an increase in the blood testosterone, LH and FSH levels, while MF monotherapy in this case was ineffective [434]. Quite unexpectedly, the authors did not find a significant effect of MF monotherapy on metabolic parameters, despite the improvement in patients’ insulin sensitivity [434].
Since MF reduces body weight in obese patients, and obesity is associated with androgen deficiency and impaired spermatogenesis [411,415,422,439,440,441,442,443], there is every reason to believe that one of the mechanisms of the improving effect of MF on male reproduction may be the normalization of adipokine status. This is supported by evidence of a positive effect of bariatric surgery on the blood levels of testosterone, LH and FSH in obese and MetS men via reduction in the adipose tissue mass and normalization in the energy metabolism [444]. Normalization of body weight in the late adolescence is important for normal puberty, since overweight and obesity during this period lead to impaired testicular function in the reproductive age [445]. Therefore, it is worthwhile to evaluate the possibility of using MF therapy for prevention of reproductive disorders in adolescents with obesity and MetS.
At the same time, there are studies showing a decrease in testosterone levels in men with T2DM and obesity during MF therapy [432,433]. Turkish scientists found that treatment of such patients with MF (1700 mg/day, three months) led to an increase in SHBG levels and a decrease in free testosterone levels in the blood [432]. However, in this study, MF-treated patients with T2DM and obesity received a low-calorie diet, which could have a negative influence on the functional state of the HPG axis and lead to a decrease in testosterone production. Conflicting clinical results on the effect of MF therapy on male reproduction require further research, especially considering the encouraging results obtained in animals with experimental models of metabolic disorders, as well as the proven ability of some other antidiabetic agents to restore androgenic status in diabetic pathology and MetS [446,447].
2.3. The Experimental Studies of Metformin Effects on Male Reproductive Dysfunctions in Animal Models of Metabolic Diseases
Most experimental works point to the restorative effect of MF on spermatogenesis and testicular steroidogenesis in animals with the experimental models of MetS, DM and obesity [448,449,450,451,452,453,454,455]. Treatment of male rats with streptozotocin (STZ)-induced diabetes with MF (100 and 500 mg/kg) for 4 or 8 weeks restored the antioxidant system and redox balance in the testes, normalized the proliferative activity of testicular somatic and germinal cells, prevented the DM-induced instability of their genome, thereby exerting an antigenotoxic effect [448]. The effect of MF was dose-dependent, and this drug, even at a relatively high dose, did not cause genotoxic and cytotoxic effects on the testicular cells of both diabetic and control animals [448].
The treatment of male Wistar rats with severe T2DM with pioglitazone (1 mg/kg) and low-dose MF (50 mg/kg) for four weeks resulted in a decrease in defective sperm and increased the caudal sperm count [449]. The treatment of male Sprague-Dawley rats with STZ-induced DM with pioglitazone alone did not prevent destructive changes in the testes, while MF was effective in this regard [456], which indicates a key role of MF in the restoration of spermatogenesis in combined therapy with pioglitazone and MF.
The key role in the development of testicular dysfunctions and in the deterioration of spermatogenesis and steroidogenesis belongs to the activation of oxidative stress, inflammation and apoptosis in the testes. Since MF has pronounced anti-inflammatory, antioxidant and antiapoptotic effects on testicular cells, these effects may be largely due to the MF-induced restoration of spermatogenesis and steroidogenesis in experimental MetS and DM [431,450,452,453,454,457]. The treatment of male Sprague-Dawley rats with MetS using MF (100 mg/kg/day, 8 weeks) weakens the apoptotic and pro-inflammatory processes in the testes and, thereby, increases the number of spermatogonia, Sertoli and Leydig cells and motile spermatozoa, decreases the number of small, atrophic and distorted seminiferous tubules, and improves the morphology of the seminiferous tubules [450]. MF also increases the blood testosterone levels, which are significantly reduced in diabetes, and also normalizes the blood levels of insulin, leptin, and estrogens [450].
A pronounced anti-inflammatory, antioxidant and antiapoptotic effect of MF was demonstrated in the testes of Sprague-Dawley rats with STZ-induced DM, which were treated with MF at a daily dose of 300 mg/kg/day for 4 weeks [452,457]. The anti-inflammatory effect of MF was due to a reduction in the expression of the inflammatory factors NF-κB, tumor necrosis factor-α (TNFα) and interleukin-1β, increased in DM, as well as the restoration of the expression interleukin-10 with anti-inflammatory activity [452]. The antioxidant effect was based on a partial restoration of the expression of the main antioxidant enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase, a decrease in the level of malonic aldehyde, the marker of oxidative stress, as well as a normalization of the expression of Nrf2 factor, which controls the expression of the enzymes protecting the cell from reactive oxygen species. The antiapoptotic effect of MF included a decrease in the DM-induced expression of proapoptotic protein p53 and the activity of caspases-3 and -8, as well as the restoration of the expression of antiapoptotic protein Bcl-2 and its ratio with proapoptotic protein Bax, reduced in diabetes [452]. In MF-treated diabetic rats, an increase in the blood and testicular levels of testosterone, a normalization of the number of the Leydig cells, an improvement in sperm morphology, a decrease in sperm nuclear DNA fragmentation and restoration of the expression and activity of the transport protein StAR and steroidogenic enzymes, such as the cytochrome P450scc (CYP11A1) and the dehydrogenases 3β-HSD and 17β-HSD were shown [452,457]. MF also increased the testicular levels of the androgenic and LH receptors, reduced in diabetes, indicating restored sensitivity of testicular cells to androgens and gonadotropins [452].
The antioxidant effect of MF was critical for its restorative effect on spermatogenesis and steroidogenesis in C57BL/6 mice with MetS induced by a HFD and cholesterol-rich diet [431]. A high content of cholesterol in food led to an increase in intratesticular cholesterol concentration, provoked the deposition of lipids in the seminiferous tubules, and impaired the morphology of the Leydig cells, stimulating the ER stress and apoptosis, culminating in reduction of testosterone synthesis [458,459]. Eight-week administration of MF led to a decrease in atherogenic cholesterol levels in the blood and lowered the cholesterol level in the testes, restoring intratesticular testosterone levels, which were decreased in MetS. Along with this, in MF-treated mice, the expression of 17β-HSD was restored [431]. The 17β-HSD catalyzes the final stage of testicular steroidogenesis, the conversion of androstenedione to testosterone. Androstenedione is a precursor of not only testosterone, but also estradiol, and its accumulation in the testes in obesity and MetS, due to the weakening of 17β-HSD activity, can enhance the synthesis of estrogens [460]. Using the cell cultures, it was shown that MF is able to suppress both the basal and insulin-stimulated expression of aromatase, which converts androgens to estrogens, and thereby restore the testosterone/estradiol ratio and improve spermatogenesis [244,246]. Thus, MF triggers several mechanisms, which lead to the normalization of the balance of sex steroid hormones in the testes in metabolic disorders.
MF restores the functions of testicular cells and spermatogenesis in rats with testicular ischemia/reperfusion caused by both clipping of the left testicular artery and vein [461,462] and the testicular torsion and deformity [451]. In the case of clipping of the testicular artery and vein, MF (100 mg/kg) and its combination with melatonin restored the activity of superoxide dismutase in the testes, which was reduced in ischemia/reperfusion, and normalized the activity of myeloperoxidase and the malonic aldehyde levels [461,462]. In the case of testicular torsion, in the testes, MF reduced the malonic aldehyde levels and inhibited the activity of caspase-3, a key enzyme of apoptosis. The antioxidant effect of MF in testicular cells was detected as early as 4 h after testicular injury [451].
Along with MF monotherapy, the combinations of MF with different natural antioxidants, including honey [463] and Malaysian propolis [453,464], restored the testicular function and hormonal parameters of the male HPG axis in diabetic animals. Some compounds with antioxidant and antiapoptotic activity, but different from MF molecular mechanisms, for example, l-carnitine, may be effective [465]. There is a question in this regard, for which mechanisms determine the antioxidant and antiapoptotic effects of MF and its restorative effect on spermatogenesis and testicular steroidogenesis? There is reason to believe that AMPK-dependent pathways are the most important, since AMPK is undoubtedly the main target of MF in the testes. It is important to note that in DM, MetS and testicular ischemia-reperfusion, one of the triggers of impaired spermatogenesis and steroidogenesis is a decrease in the activity of testicular AMPK. In 2012, Ana Hurtado de Llera and colleagues showed that pharmacological inhibition of AMPK in the testes dramatically reduces the percentages of motile and rapid spermatozoa [466]. There is a lot of evidence that AMPK regulates the growth and differentiation of the Sertoli and Leydig cells, controls the motor activity of spermatozoa and their acrosomal reaction. Moreover, AMPK is responsible for antioxidant activity and production of reactive oxygen species in the testicular somatic and germ cells, and determines the metabolic processes in them, including lipid metabolism [194,467,468,469,470]. Accordingly, the normalization of AMPK-dependent pathways in the testes under the influence of MF may be the main mechanisms of its action on improving male reproduction in DM and other metabolic diseases.
It can be assumed that the normalization of AMPK signaling in the testes may be due not only to the direct effect of MF on testicular AMPK activity, but also due to the restoration of the leptin signaling pathway in the testes of diabetic rats, especially since AMPK is also one of the leptin targets [471,472], in particular in the testes [473]. The MF treatment (4 weeks, 120 mg/kg) of male Wistar rats with HFD/STZ-induced T2DM and severe hyperleptinemina led to a normalization of the blood and testicular levels of leptin and an increase in the number of testicular leptin receptors [455]. The two-week MF treatment (500 mg/kg) of albino mice with STZ-induced T1DM also improved the leptin signaling pathway in the testes, increasing the expression of ObRb, functionally active isoform of leptin receptor, in the Leydig cells, primary spermatocytes and round spermatids [454]. The improvement of the testicular leptin signaling pathway was accompanied by the restoration of the steroidogenic genes expression, including the cholesterol-transporting protein StAR, an increase in the sensitivity of the Leydig cells to hCG, and a weakening of the apoptotic processes in testicular cells [454,455].
3. Conclusions
The data presented in the review convincingly prove that MF has an improving effect on reproductive functions in men with DM and MetS.The effectiveness of MF therapy is due to a large number of different factors that must be taken into account when choosing this therapy and also when developing a strategy for using MF. Firstly, it is necessary to assess the efficiency of MF transport into the cell, which depends on the functional activity of the organic cation transporters and can be disrupted by inactivating mutations in their genes. The presence of certain mutations leads to a loss of responsiveness to MF and makes the use of MF therapy meaningless. Secondly, MF therapy is more effective in the severely overweight and obese patients with IR, compensatory hyperinsulinemia, impaired glucose tolerance, and dyslipidemia. This is not surprising, since the clinical effect of MF therapy is due to an improvement in insulin sensitivity, a decrease in the adipose tissue mass, and restoration of the glucose and lipid metabolism.
Since some of MF targets overlap well with those of leptin, the assessment of leptin status in males with reproductive disorders may also be important. As a result, leptin resistance, both systemic and in the testes, as well as the changes in the hypothalamic leptin signaling pathways, negatively affecting the production of GnRH, can become factors that will determine the effectiveness of MF therapy. In this regard, it should be noted that the central mechanisms of action of MF, which easily penetrates the CNS and improves the metabolism of the neuronal and glial cells, still remain underestimated. By acting on the CNS, MF restores the signaling networks of the hypothalamus and the other brain regions that are involved in the control of steroidogenesis and spermatogenesis and undergo significant compensatory and pathological changes in metabolic and endocrine disorders.
A unique feature of MF is the multiplicity of molecular mechanisms of its action on target cells, which include direct or indirect regulation of the AMPK-, calcium- and cAMP-dependent signaling pathways, as well as the mitogen-activated protein kinase (MAPK) cascade and the insulin receptor substrate (IRS)/phosphatidylinositol 3-kinase (PI 3-K)/Akt pathway. As a result, MF controls not only energy and metabolic processes in the cell, but also the processes of growth, differentiation, apoptosis, inflammation, and endoplasmic reticulum stress. At the same time, most of the regulatory effects of MF are based largely on its modulating and normalizing influence on intracellular signaling cascades than on their prolonged stimulation or suppression. Depending on the functional state of the IRS/PI 3-K/Akt pathway, MF can either prevent its hyperactivation, which is especially important for its antitumor effect, or, on the contrary, restore its reduced activity, improving the survival of target cells and their sensitivity to insulin and leptin. As expected, MF therapy affects the responsiveness of hypothalamic neurons, pituitary gonadotrophs, and testicular cells to the hormones, growth factors, adipokines and cytokines.
The use of MF in combination with insulin and other drugs has great potential. In men with metabolic and endocrine disorders, the combined therapy not only allows to increase the efficiency and pattern of the effects of MF on the HPG axis, but also to reduce the pharmacological doses of drugs, including MF, thus avoiding possible side effects of high-dose drug administration, including the undesirable effect of MF on the functioning of the gastrointestinal tract.
The entry is from 10.3390/ph14010042This entry is offline, you can click here to edit this entry!
