Radiotherapy remains one of the contemporary cornerstones of cancer treatment in the neoadju-vant, curative, adjuvant and palliative settings, either in isolation or as a multimodal approach. Moreover, recent advances in targeted immune checkpoint therapy have firmly established im-munotherapy as the fourth pillar in cancer therapy alongside surgery, chemotherapy and notably radiotherapy. There is emerging evidence to suggest both radioresistance and reduced efficacy of immune checkpoint blockade (ICB) are potentiated by the tumour microenvironment (TME) and in fact modulating aspects of this immunosuppressive milieu is instrumental to unlocking an-ti-tumour immunity.
- radiotherapy
- immunotherapy
- dosing
- timing
- curative
- upper gastrointestinal cancers
1. Introduction
After years of effort to harness the immune system for the treatment of cancer, the advent of antibodies which target ‘immune checkpoints’, including programmed cell death protein 1 (PD-1) and cytotoxic lymphocyte antigen 4 (CTLA-4), has increased interest in immunological aspects of conventional therapies. These immune checkpoint inhibitors (ICIs), including pembrolizumab, tislelizumab, nivolumab (anti PD-1) and ipilimumab (anti CTLA-4), have led to dramatic and durable clinical responses in diverse cancers [1]. Cancers of the upper gastrointestinal tract (UGI) are common globally and account for a disproportionately high incidence of cancer-related mortality. In 2019, UGI malignancies accounted for 9% of cancer diagnoses and 13.5% of cancer related deaths worldwide [2]. The response rates for clinically approved ICIs in melanoma and non-small cell lung cancer are approximately 20–40% [2]; however, in UGI cancers, this decreases to 10–15% and ICIs are therefore largely confined to salvage treatment of advanced disease, with its use in the neoadjuvant and curative setting confined to select cases [3].
Radiotherapy has been one of the pillars for the management of neoplastic burden in cancer patients for over a century. It is used as a treatment modality in approximately 50% of cancer patients in the neoadjuvant, adjuvant, curative or palliative settings [4]. Tri-modality treatment of surgery, chemotherapy and radiotherapy is the standard of care in oesophageal adenocarcinoma (OAC) and oesophageal squamous cell carcinoma (OSCC), while in gastric cancer (GC) adjuvant chemotherapy use depends on surgical margins and the extent of lymph node dissection [5]. Radiation triggers DNA damage-induced cell death in cancer cells but can also modify the antigenicity and the adjuvanticity of tumours. This is by activating cytosolic DNA sensors, inducing immunogenic cell death, enhancing neoantigen expression and modulating the tumour microenvironment (TME) [6]. Therefore, combining immuno-oncology approaches with radiation could boost response to ICI in UGI cancers (Figure 1). In this review, we describe the mechanistic rationale for combining immunotherapy and radiotherapy, clinical trials of radio-immunotherapy in gastroesophageal malignancies and current strategies to optimise radiation regimen efficacy while minimising toxicity.
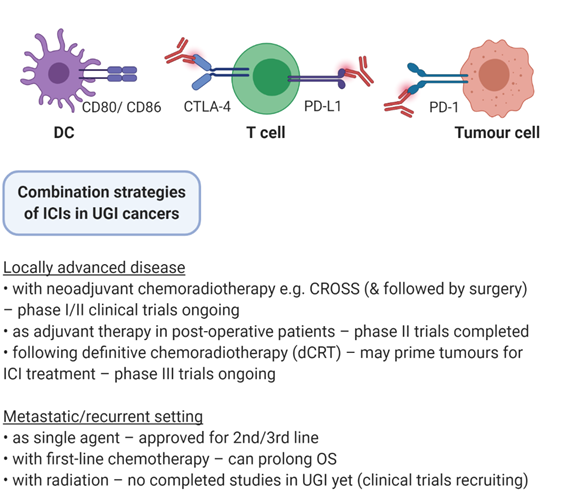
Figure 1. Combination strategies of ICIs in UGI cancers. Immune checkpoint inhibitors (ICIs) are under investigation in a variety of settings in upper gastrointestinal (UGI) cancers, including in conjunction with surgery, chemotherapy, radiation therapy, and multimodal combinations.
2. Clinical Trials of Radiation and Immunotherapy in UGI Cancer
2.1. Single Agent Immunotherapy
Single agent ICI trials in UGI cancers have delivered modest results. The ATTRACTION-2 phase III trial found that nivolumab (anti-PD-1) improved overall survival (OS; 5.3 vs. 4.1 months in the placebo group, p < 0.0001) in heavily pretreated GC or gastroesophageal junction cancer (GEJC) regardless of PD-L1 expression [7]. The KEYNOTE-059 phase II study evaluated pembrolizumab (anti-PD-1) versus chemotherapy in previously treated GC or GEJC, with the objective response rate (ORR) of 11.6%, with a longer median duration of response in PD-L1+ patients (16.3 vs. 6.9 months) [8]. Based on these results, in 2018 the FDA granted accelerated approval to pembrolizumab in the third line treatment of recurrent GC or GEJC that overexpresses PD-L1 with a Combined Positive Score [CPS] ≥ 10), as determined by a U.S. Food and Drug Administration (FDA)-approved test, with disease progression after one or more prior lines of systemic therapy as identified in KEYNOTE-181. Clinical efficacy of ICIs in OSCC is slightly more encouraging, with KEYNOTE 181, a phase III trial reporting that as second line treatment, the median OS with pembrolizumab vs. chemotherapy was similar in the intention to treat(ITT) group (7.1 vs. 7.1 months) and longer in the SCC (8.2 vs. 7.1 months) and PD-L1 CPS ≥10 groups (9.3 vs. 6.7 months) [9]. A trend was observed favouring responses in OSCC, forming the basis for the 2019 FDA approval of pembrolizumab in the second line treatment of metastatic PD-L1+ OSCC. More recently, the ATTRACTION-3 phase III trial of second line nivolumab vs. chemotherapy confirmed this OS benefit (10.9 months vs. 8.4 months, p = 0.019), further supporting the place of PD-1 inhibition in metastatic OSCC [10]. Approved indications of immunotherapy in gastroesophageal cancer are therefore confined to the second- or third-line treatment of metastatic disease. However, more recent data from the CheckMate-649 and KEYNOTE-590 studies indicate that the addition of nivolumab (median OS: 14.4 vs. 11.1, HR 0.71, p < 0.0001) or pembrolizumab (median OS: 12.4 vs. 9.8, HR 0.73, p < 0.0001) to first line chemotherapy in metastatic UGI cancers can prolong overall survival [11][12]. This suggests that combining ICIs with more traditional treatment modalities can improve outcomes in gastroesophageal cancers.
Prospective clinical data of combining immunotherapy and radiation are in a nascent phase; phase III trials have not been published outside of prostate and non small cell lung cancer (NSCLC). The phase III PACIFIC trial investigated durvalumab (anti PD-L1) following chemoradiotherapy in locally advanced NSCLC [13]. Compared to placebo, durvalumab improved overall survival (28.3 vs. 16.2 months) and both arms had similar rates of treatment related adverse events. A phase III trial in metastatic castration resistant prostate cancer found no benefit of ipilimumab (anti CTLA-4) following a single 8Gy dose of radiation to up to five bone metastases [14]. In gastroesophageal cancers, trials are in early stages and the majority are ongoing. Clinical investigation focuses on three settings: disease treatable by surgical resection, definitive chemoradiotherapy and palliative treatment in the metastatic setting with timing of delivery and ideal combination multimodal therapies yet to be elucidated.
2.2. Locally Advanced Disease
Locally advanced, nonmetastatic UGI cancers are optimally treated by surgical resection. This is accompanied by the CROSS neoadjuvant chemoradiotherapy regimen consisting of carboplatin, paclitaxel and 41.4 Gy external beam radiotherapy (EBRT) [15]. However, only 15% of patients display a pathologic complete response (pCR) and trials are investigating if the addition of ICIs can improve response rates and patient outcomes (Table 1). One example is phase I trial (NCT03044613) of pembrolizumab (anti PD-1) in combination with the CROSS regimen in stage II/II OAC, OSCC or GEJC. Primary endpoints are pCR rate and treatment related adverse events (TRAEs). Preliminary results for the first 10 patients show an encouraging pCR rate of 40%, with acceptable toxicity and no delays in surgery [16]. A larger (n = 28) phase II trial of pembrolizumab in OSCC has reported a pCR rate of 46.1%, with 82% of patients surviving at 12 months [17]. However, 2/28 patients did not undergo surgery and two treatment related deaths were reported due to acute lung injury emphasising the need for TRAE monitoring. Another approach is the use of adjuvant ICIs in postoperative patients to better control micro-metastatic disease. A phase II trial has evaluated durvalumab (anti PD-L1) in OAC or GEJC previously treated with external beam radiotherapy (EBRT) with residual disease following tri-modality treatment of surgery and chemoradiation (NCT02639065). Early results indicate that adjuvant durvalumab is safe with few dose limiting toxicities [18]. Relapse free survival in this single arm study was 79.2%, which compares favourably to the historical rate of 50%. These encouraging results further underscore the need for more data in an adjuvant setting.
Table 1. Ongoing clinical Trials of radiation and immunotherapy in operable disease.
|
Identifier |
Phase |
N |
Disease Setting |
Treatment |
Radiation |
Primary Endpoint |
|
NCT03792347 |
I |
20 |
Stage II/III OSCC |
Pembrolizumab, carboplatin, and paclitaxel |
41.4Gy in 23 fractions |
TRAEs |
|
NCT02844075 |
II |
18 |
Stage II/III OSCC |
Pembrolizumab, carboplatin, and paclitaxel |
41.4Gy in 23 fractions |
pCR rate |
|
NCT03257163 |
II |
40 |
Stage II/III dMMR or EBV+ GC |
Neoadjuvant pembrolizumab |
Conventional Fractionation |
RFS |
|
Adjuvant Capecitabine and pembrolizumab |
||||||
|
NCT03064490 |
II |
38 |
Stage II/III GC or OAC |
Pembrolizumab, carboplatin, and paclitaxel |
41.4Gy in 23 fractions |
pCR rate |
|
NCT02730546 |
I/II |
68 |
Stage II/III GC or GEJC |
Pembrolizumab, carboplatin, and paclitaxel |
41.4Gy in 23 fractions |
pCR rate |
|
PFS |
||||||
|
NCT03044613 |
I |
25 |
Stage II/III OAC, OSCC or GEJC |
Nivolumab and carboplatin and paclitaxel |
41.4Gy in 23 fractions |
TRAEs |
|
NCT03776487 |
I/II |
30 |
Stage II/III GC or GEJC |
FOLFOX and Nivolumab and ipilimumab followed by surgical resection |
50 gy 25 fractions |
TRAEs |
|
NCT02962063 |
II |
35 |
Stage II/III GEJC and GC |
Neoadjuvant Durvalumab and mFOLFOX Adjuvant durvalumab |
50Gy in 28 fractions |
TRAEs |
|
pCR rate |
||||||
|
NCT04159974 |
II |
56 |
Stage II/III OAC or GEJC |
Durvalumab, carboplatin and paclitaxel |
41.4Gy in 23 fractions |
pCR rate |
|
TRAEs |
||||||
|
NCT02639065 |
II |
23 |
Stage II/III OAC or GEJC with residual disease |
Durvalumab |
41.4Gy in 23 fractions |
TRAEs |
|
DLTs |
||||||
|
NCT03490292 |
I/II |
24 |
Stage II/III OSCC or OAC |
Avelumab and Carboplatin, paclitaxel |
41.4Gy in 23 fractions |
DLT |
|
pCR |
Abbreviations: TRAEs, treatment related adverse effects; pCR, pathological complete response; DLT, dose limiting toxicity; OAC, oesophageal adenocarcinoma; OSCC, oesophageal squamous cell carcinoma; GEJC, gastroesophageal junction adenocarcinoma; dMMR, deficient mismatch repair; Gy, Gray; RFS, relapse free survival.
2.3. Definitive Chemoradiotherapy
Definitive chemoradiotherapy (dCRT) is an alternative standard of care in OSCC and is also employed in localised OAC deemed unsuitable for surgery. A 50.4Gy EBRT is delivered in 25 fractions, accompanied by 5-FU and cisplatin or FOLFOX (5FU, folinic acid and oxaliplatin). dCRT may promote immunogenic changes in tumours, priming tumours for ICI treatment to further enhance local and distant tumour control. The randomised, doubled blinded, phase III KEYNOTE-975 trial is evaluating pembrolizumab with traditional dCRT in localised but inoperable OSCC, OAC and GEJC (NCT04210115). The primary endpoints are overall survival and event free survival, and results could define a new standard of care in the dCRT setting. Other ongoing trials are evaluating dual checkpoint blockade (anti PD-1 and anti CTLA-4; NCT03437200), and sequential nivolumab and cetuximab (anti EGFR) with concomitant dCRT.
2.4. Systemic Treatment of Advanced Disease
Combining ICIs and radiation in the recurrent or metastatic setting seeks to activate the abscopal response, priming antigen specific CD8+ T cells against tumours outside the radiation field. The few radio-immunotherapy trials in metastatic UGI cancers are at an early stage and seek to investigate toxicities and mechanisms of response. One example, phase II trial of pembrolizumab and 30Gy conventional fractionated radiotherapy is recruiting patients with metastatic gastroesophageal cancers (NCT03544736). Primary endpoints aim to quantify the abscopal response; changes in CD8+ TILs at the irradiated site, and changes in MDSCs and Tregs at peripheral metastases will be measured. Another approach is a combination of nivolumab and high dose brachytherapy to deliver 16Gy over 2 fractions (NCT02642809), and durvalumab with concomitant chemoradiotherapy in the palliative setting (NCT03544736).
This entry is adapted from the peer-reviewed paper 10.3390/ijms22031071
References
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential bi-omarkers. Exp. Mol. Med. 2018, 50, 1–11.
- Maharaj, A.D.; Holland, J.F.; Scarborough, R.O.; Evans, S.M.; Ioannou, L.J.; Brown, W.; Croagh, D.; Pilgrim, C.; Kench, J.; Lipton, L.; et al. The Upper Gastrointestinal Cancer Registry (UGICR): A clinical quality registry to monitor and improve care in upper gastrointestinal cancers. BMJ Open 2019, 9, e031434.
- Kelly, R.J. The emerging role of immunotherapy for esophageal cancer. Curr. Opin. Gastroenterol. 2019, 35, 337–343.
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199.
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Primers 2017, 3, 17048.
- Zemek, R.M.; Chin, W.L.; Nowak, A.K.; Millward, M.J.; Lake, R.A.; Lesterhuis, W.J. Sensitizing the Tumor Microenvironment to Immune Checkpoint Therapy. Front. Immunol. 2020, 11, 223.
- Kang, Y.-K.; Boku, N.; Satoh, T.; Ryu, M.-H.; Chao, Y.; Kato, K.; et al. Nivolumab in patients with advanced gastric or gas-tro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538–12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471.
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEY-NOTE-059 Trial. JAMA Oncol. 2018, 4, e180013.
- Kojima, T.; Muro, K.; Francois, E.; Hsu, C.-H.; Moriwaki, T.; Kim, S.-B.; et al. Pembrolizumab versus chemotherapy as sec-ond-line therapy for advanced esophageal cancer: Phase III KEYNOTE-181 study. J. Clin. Oncol. 2019, 37 (Suppl. S4), 2.
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.-Y.; Chin, K.; et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1506–1517.
- Kato, K.; Sun, J.M.; Shah, M.A.; Enzinger, P.C.; Adenis, A.; Doi, T.; et al. LBA8_PR Pembrolizumab plus chemotherapy ver-sus chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase 3 KEYNOTE-590 study. Ann. Oncol. 2020, 31, S1192–S1193.
- Moehler, M.; Shitara, K.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; et al. LBA6_PR Nivolumab (nivo) plus chemother-apy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann. Oncol. 2020, 31, S1191.
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350.
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; van den Eertwegh, A.J.; et al. Ipilimumab versus placebo after ra-diotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 700–712.
- Weichselbaum, R.R.; Liang, H.; Deng, L.; Fu, Y.X. Radiotherapy and immunotherapy: A beneficial liaison? Nat. Rev. Clin. Oncol. 2017, 14, 365–379.
- Jones, C.M.; Spencer, K.; Hitchen, C.; Pelly, T.; Wood, B.; Hatfield, P.; et al. Hypofractionated Radiotherapy in Oesophageal Cancer for Patients Unfit for Systemic Therapy: A Retrospective Single-Centre Analysis. Clin. Oncol. (R Coll. Radiol.) 2019, 31, 356–364.
- Spaas, M.; Lievens, Y. Is the Combination of Immunotherapy and Radiotherapy in Non-small Cell Lung Cancer a Feasible and Effective Approach? Front. Med. 2019, 6, 244.
- Chakraborty, M.; Abrams, S.I.; Camphausen, K.; Liu, K.; Scott, T.; Coleman, C.N.; et al. Irradiation of Tumor Cells Up-Regulates Fas and Enhances CTL Lytic Activity and CTL Adoptive Immunotherapy. J. Immunol. 2003, 170, 6338.