Blood flow modeling consists of using computational techniques to investigate the blood flow behavior in a rapid and accurate fashion. This has become an area of extensive research due to the prevalence of cardiovascular diseases, responsible for a critical number of deaths every year worldwide, most of which are associated with atherosclerosis, a disease that causes unusual hemodynamic conditions in arteries. In the present review, the application of computational simulations by using different physiological conditions of blood flow, several rheological models, and boundary conditions, were discussed.
- atherosclerosis
- coronary arteries
- hemodynamics
- numerical methods
1. Introduction
Despite the progress done in experimental studies and blood flow measurement techniques, there are still some challenges associated with them [19]. For instance, in vitro wall shear stress (WSS) measurements are extremely difficult to perform and the velocity measurements have high associated errors. These, combined with other complications of directly measuring quantities of interest, have motivated the use of computer simulations to predict them in silico [69].
The earliest numerical detailed studies solving the flow problem in constricted tubes were conducted by Lee and Fung (1970) [70]. After that, other studies in this field conducted by Caro et al., (1971) [71], Glagov et al., (1989) [72], and Ku et al., (1985) [73] are important references in this area and should be highlighted. Ever since, CFD approaches have been progressively adopted by most researchers as the preferred technique for numerical modeling of hemodynamics. Owing to the continued growth of computational power, these have become an increasingly reliable tool for measuring biomechanical factors vital for clinical decision-making and surgical planning. However, the proper selection of the flow boundary conditions has to be done, otherwise, the findings can be considered uncertain, weak, and unrealistic [74]. In this regard, the different geometries, boundary conditions, and flow characteristics applied by some researchers in the last ten years are summarized in Table 1.
Table 1. Numerical studies of hemodynamics and the respective assumptions for numerical simulations.
| Geometry | Schematic Representation | Modeling Approaches | Fluid | Boundary Conditions | Authors | ||
|---|---|---|---|---|---|---|---|
| Wall | Inlet | Outlet | |||||
| Idealized | 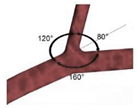 |
Laminar | Non-Newtonian (Carreau-Yasuda) | Rigid | Time-dependent velocity profile | Zero gauge pressure | Kashyap et al., (2020) [6] |
| Idealized | 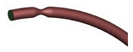 |
Laminar | Newtonian | Rigid | Time-dependent mass flow profile | Zero surface tension | Biglarian et al., (2019) [75] |
| Idealized |  |
Laminar | Non-Newtonian (Cross model) | Rigid and Flexible | Constant inlet velocity | Constant pressure outlet (10 kPa) | Mulani et al., (2015) [57] |
| Idealized |  |
Laminar | Newtonian | Rigid and Flexible | Time-dependent flowrate profile | Time-dependent pressure profile | Wu et al., (2015) [58] |
| Idealized | 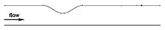 |
Laminar | Newtonian | Rigid | Constant inlet velocity (fully developed parabolic profile) | Constant pressure outlet (13 kPa) | Kenjereš et al., (2019) [76] |
| Idealized |  |
Laminar | Newtonian | Rigid | Constant inlet velocity | Zero gauge pressure | Carvalho et al., (2020) [47] |
| Idealized |  |
k-ω turbulent model | Non-Newtonian (Carreau model) | Rigid | Spiral boundary conditionwith a parabolic velocity profile | Zero gauge pressure | Kabir et al., (2018) [77] |
| Idealized |  |
k-ω turbulent model (SST) | Non-Newtonian (Carreau model) | Rigid | Time-dependent velocity profile | Zero gauge pressure | Carvalho et al., (2020) [42] |
| Idealized |  |
k-ω turbulent model (SST) | Non-Newtonian (Carreau model) | Rigid | Time-dependent velocity profile | Zero gauge pressure | Carvalho et al., (2020) [59,78] |
| Idealized |  |
N.A1 | Newtonian | Flexible | Time-dependent velocity profile | Time-dependent pressure profile | Jahromi et al., (2019) [79] |
| Idealized |  |
Laminar | Newtonian | Rigid | Time-dependent velocity profile | Flow partition implied in Murray’s law | Doutel et al., (2018) [11] |
| Patient-specific | 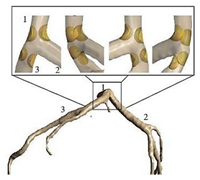 |
Laminar | Non-Newtonian (Generalized power-law model) and Newtonian | Rigid | Time-dependent flow rate profile | Time-dependent pressure profile | Chaichana et al., (2012) [60] |
| Patient-specific |  |
Laminar | Non-Newtonian (Carreau model) | Rigid | Time-dependent velocity profile | Time-dependent pressure profile | Liu et al., (2015) [80] |
| Patient-specific |  |
Laminar | Newtonian | Rigid and Flexible | Time-dependent pressure profile | Parabolic velocity profile | Siogkas et al., (2014) [81] |
| Patient-specific |  |
N.A | Newtonian | Rigid | Time-dependent pressure profile | Constant pressure outlet (9.85 kPa) | Zhao et al., (2019) [82] |
| Patient-specific |  |
Laminar | Non-Newtonian (Carreau model) | Rigid | Time-dependent velocity profile | Flow partition implied in Murray’s law | Pandey et al., (2020) [43] |
| Patient-specific |  |
Laminar | Non-Newtonian (Carreau model) | Rigid | Various time-dependent velocity profiles | Flow partition implied in Murray’s law | Rizzini et al., (2020) [74] |
| Patient-specific |  |
N.A | Non-Newtonian (Power-law model) | Rigid | Time-dependent velocity profile | Pressure outlet (N.A) | Zhang et al., (2020) [83] |
| Patient-specific |  |
k-ω turbulent model (SST) | Non-Newtonian (Bird-Carreau model) | Rigid | Time-dependent velocity profile | Constant pressure outlet (10 kPa) | Kamangar et al., (2019) [64] |
| Patient-specific |  |
Laminar | Newtonian | Rigid | Time-dependent flow rate profile | Two-Element Windkessel Model | Lo et al., (2019) [84] |
| Patient-specific and Idealized |  |
Laminar | Newtonian and Non-Newtonian (Carreau model) | Rigid | Constant inlet velocity and Time-dependent velocity profile | N.A | Doutel et al., (2019) [85] |
| Patient-specific and Idealized |  |
k-ω turbulent model (SST) | Non-Newtonian (Carreau model) | Rigid | Time-dependent velocity profile | Outflow condition | Mahalingam et al., (2016) [86] |
| Patient-specific and Idealized |  |
N.A | Non-Newtonian (Carreau model) | Rigid | Time-dependent velocity profile | Constant pressure outlet (10 kPa) | Rabbi et al., (2020) [87] |
| Patient-specific and Idealized |  |
Laminar | Newtonian | Rigid | Constant inlet mass flow and Time-dependent flow rate | Zero gauge pressure | Malota et al., (2018) [88] |
In general, from the above-mentioned investigations, it can be seen that, regardless of the type of geometry, the majority of authors consider that the blood is a non-Newtonian fluid, usually approximated by the Carreau model, with a laminar behavior. Regarding the boundary conditions, in most cases, the wall is considered rigid, and at the inlet, a pulsatile velocity is applied. At the outlet, the condition set mainly depends on the study, but either the default conditions are maintained, or pressures are applied, time-dependent or constant values. In the following section, the main observations drawn from these studies are presented.
2. Concluding remarks and future perspectives
Although huge advancements have been made in imaging techniques to obtain patient-specific images, this step is time-consuming, and it is still a challenging task for all researchers. For this reason, nowadays, idealized models continue to be widely used by researchers, since these allow to obtain important and relevant results, without requiring much computational time and without the need to collect the medical images, which is highly time-consuming. In this regard, a promising study was proposed by Doutel et al., (2018) [1] wherein artificial, but realistic stenosis can be generated.
It was also noted that, although the modelling of blood as a Newtonian fluid is a good approximation for large vessels with high shear rates, the assumption of non-Newtonian behavior of blood flow has been increasingly used in the presence of stenosis. From the overall studies, the most used models are the Carreau and the Carreau-Yasuda, and these have also been indicated as the most appropriate to simulate the blood rheology by Razavi et al., (2011) [2]. Nevertheless, currently, one cannot say which is the right model, because there is not yet enough evidence in the literature to prove which model fully expresses the complex nature of blood rheology and its dependence on many biological factors [3]. Accordingly, it is of great importance to obtain proper models for CFD analysis that take into account the non-Newtonian behavior of blood. For this purpose, more experimental studies are needed. Regarding the boundary conditions, few studies have evaluated the impact of using different inlet and outlet boundary conditions [4][5], and therefore, it would be interesting, in future studies, to investigate what are the profiles more adequate to study the blood flow behavior in coronary arteries.
Despite the great efforts that have been made so far, the blood has been mainly modeled as a single-phase fluid. However, blood is a mixture of plasma, red blood cells, white blood cells, and platelets. Therefore, the consideration of multiphase models is of great importance when modeling atherosclerotic lesions. Although some studies have already applied these models [6][7][8][9], the research is still in the beginning. Moreover, it should be also noted that the use of these models is a promising option for studying nanoparticle-mediated targeted drug delivery treatment of atherosclerosis. In this context, a promising study was conducted by Zhang et al., (2020)[7]. The authors used an Eulerian-Lagrangian approach coupled with FSI to investigate the impact of plaque morphology on magnetic nanoparticles targeting under the action of an external field.
Due to the continuous improvements acquired in computational methods, in the following years more amazing and complex hemodynamic studies will be performed. The work of Zhao et al. (2019) [10] should be highlighted since their numerical approach has a great potential to achieve more realistic simulations. They have simulated 4D hemodynamic profiles of time-resolved blood flow. The results proved that these simulations can provide extensive information about blood flow, both qualitatively and quantitatively that may be advantageous for future investigations of clinical diagnosis and treatment of atherosclerosis.
To conclude, although computational methods have been extensively used for atherosclerosis investigations in recent years, they are expected to become more popular and more effective to simulate the blood flow in the cardiovascular system, and consequently, they will promote medical innovation at an affordable cost. However, to this end, active collaborations between engineers and medical staff are needed to assure the successful application of this technique in atherosclerosis treatment.
Reference (Editors will rearrange the references after the entry is submitted)
This entry is adapted from the peer-reviewed paper 10.3390/fluids6020053
References
- E. Doutel; J. Carneiro; J.B.L.M. Campos; J.M. Miranda; Artificial stenoses for computational hemodynamics. Applied Mathematical Modelling 2018, 59, 427-440, 10.1016/j.apm.2018.01.029.
- A. Razavi; Ebrahim Shirani; M.R. Sadeghi; Numerical simulation of blood pulsatile flow in a stenosed carotid artery using different rheological models. Journal of Biomechanics 2011, 44, 2021-2030, 10.1016/j.jbiomech.2011.04.023.
- Jack Lee; Nicolas P. Smith; The Multi-Scale Modelling of Coronary Blood Flow. Annals of Biomedical Engineering 2012, 40, 2399-2413, 10.1007/s10439-012-0583-7.
- Maurizio Lodi Rizzini; Diego Gallo; Giuseppe De Nisco; Fabrizio D'ascenzo; Claudio Chiastra; Pier Paolo Bocchino; Francesco Piroli; Gaetano Maria De Ferrari; Umberto Morbiducci; Does the inflow velocity profile influence physiologically relevant flow patterns in computational hemodynamic models of left anterior descending coronary artery?. Medical Engineering & Physics 2020, 82, 58-69, 10.1016/j.medengphy.2020.07.001.
- Alina G Van Der Giessen; Harald C. Groen; Pierre-André Doriot; Pim J. De Feyter; A.F.W. Van Der Steen; Frans N. Van De Vosse; J.J. Wentzel; F. J. H. Gijsen; The influence of boundary conditions on wall shear stress distribution in patients specific coronary trees. Journal of Biomechanics 2011, 44, 1089-1095, 10.1016/j.jbiomech.2011.01.036.
- Violeta Carvalho; Nelson Rodrigues; Ricardo Ribeiro; Pedro F. Costa; José C. F. Teixeira; Rui A. Lima; Senhorinha F. C. F. Teixeira; Hemodynamic study in 3D printed stenotic coronary artery models: experimental validation and transient simulation. Computer Methods in Biomechanics and Biomedical Engineering 2020, 2, 1-14, 10.1080/10255842.2020.1842377.
- Xuelan Zhang; Mingyao Luo; Erhui Wang; Liancun Zheng; Chang Shu; Numerical simulation of magnetic nano drug targeting to atherosclerosis: Effect of plaque morphology (stenosis degree and shoulder length). Computer Methods and Programs in Biomedicine 2020, 195, 105556, 10.1016/j.cmpb.2020.105556.
- Wei-Tao Wu; Yubai Li; Nadine Aubry; Mehrdad Massoudi; James F. Antaki; Numerical Simulation of Red Blood Cell-Induced Platelet Transport in Saccular Aneurysms. Applied Sciences 2017, 7, 484, 10.3390/app7050484.
- Abdulrajak Buradi; Sumant Morab; Arun Mahalingam; EFFECT OF STENOSIS SEVERITY ON SHEAR-INDUCED DIFFUSION OF RED BLOOD CELLS IN CORONARY ARTERIES. Journal of Mechanics in Medicine and Biology 2019, 19, 1950034, 10.1142/s0219519419500349.
- Yinghong Zhao; Jie Ping; Xianchao Yu; Renyuan Wu; Cunjie Sun; Min Zhang; Fractional flow reserve-based 4D hemodynamic simulation of time-resolved blood flow in left anterior descending coronary artery. Clinical Biomechanics 2019, 70, 164-169, 10.1016/j.clinbiomech.2019.09.003.
