The ElectroKinetic Remediation Technology (EKRT) is an electrochemical approach that is applied for the remediation of contaminated soils. Electrochemical approaches have gained prominence thanks to the many possible applications and their proven effectiveness. This is particularly evident in the case of inorganic/ionic contaminants, which are not subject to natural attenuation (biological degradation) and are difficult to treat adequately with conventional methods.
- ElectroKinetic Remediation
- environmental contaminations
1. Introduction
The EKRT approach consists in the application of an electric potential gradient to induce a low electric current across a portion of the contaminated soil to be treated, using electrodes suitably positioned in the subsurface [1]. It can be applied both in situ and ex situ and allows high efficiency even if performed in soils with low permeability [2][3], as the applied electric field can easily reach contaminants embedded deep in the subsurface, which other technologies are unable to reach [4][5].
Depending on the intensity of the resulting electric current and the characteristics of the system (salt content, moisture, soil composition, etc.), different physical, chemical and electrochemical processes are induced. These may allow a substantial migration of species through the soil and towards the electrode wells (housing for the electrodes and the electrolyte solution) from where they can eventually be removed [6] by electrodeposition, adsorption, precipitation, or co-precipitation on the electrodes [7], or simply by removing the contaminated electrolyte solution (which can then be treated and reused [8]).
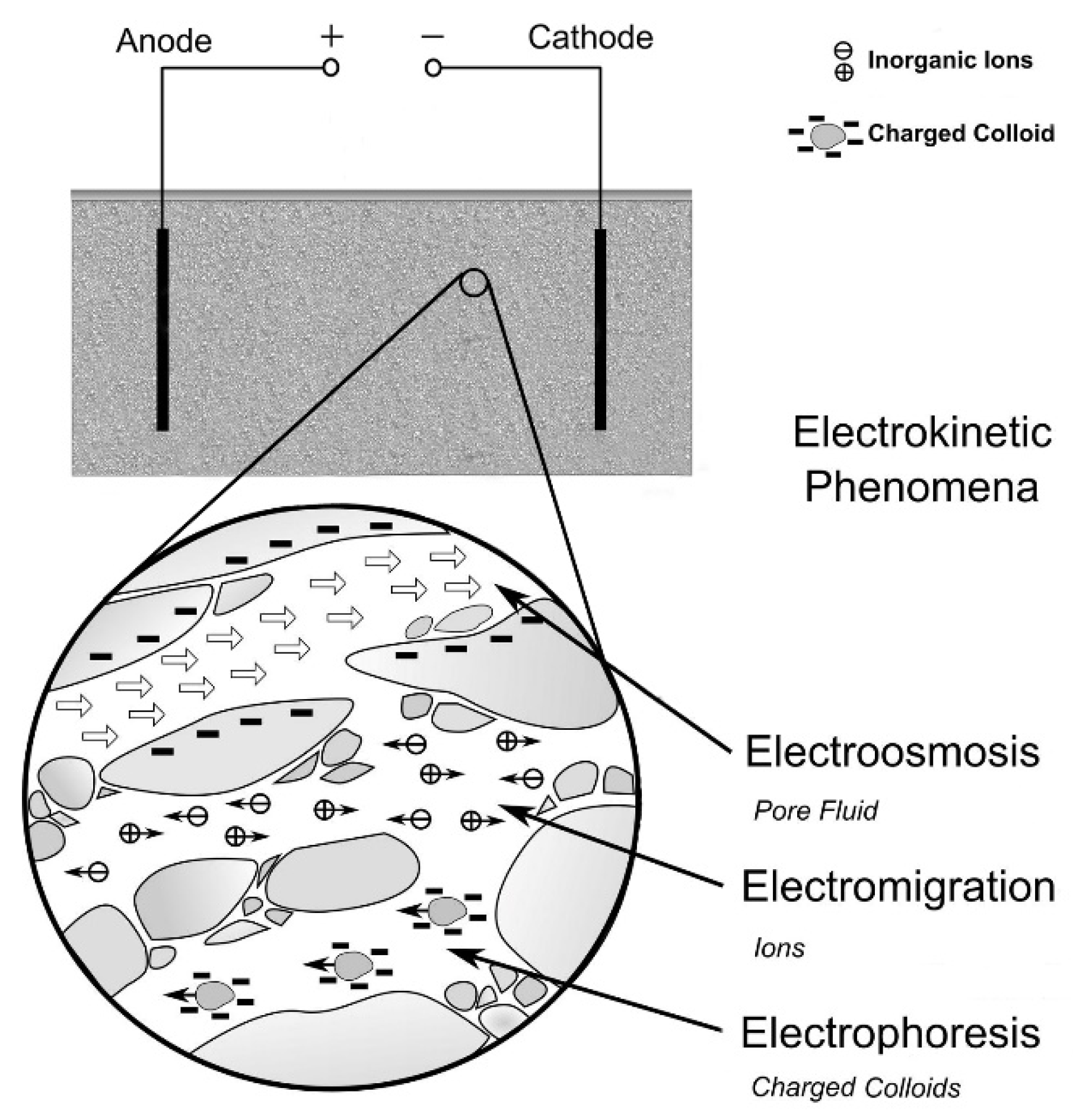
The most relevant phenomena induced in a soil by the applied electric field are listed below[9] and graphically illustrated in Figure 1:
- electroosmosis, i.e., the displacement of the solution naturally present in the soil;
- (electro)migration of electrically charged species;
- electrolysis, a process that occurs on the surfaces of electrodes, generally at the expense of water (decomposition reactions);
- electrophoresis, that is the transport of charged particles of colloidal size present within a stationary fluid, due to the application of an electric gradient.
Figure 1. Detail of the main mechanisms occurring during an ElectroKinetic Remediation Technology (EKRT) remediation. Adapted from [10].
Moreover, since soils are usually characterized by a high ionic resistance, an increase in temperature is normally observed (Joule–Thompson effect) [11]; the dissipated energy is proportional to the square of the current flowing through the soil. It is worth noting that, in the presence of volatile pollutants, this can cause significant environmental problems.
Under the action of the electric field generated between electrodes, anions and cations move toward the anodes and the cathodes, respectively (electromigration). In addition, the anodic oxidation of water generates an acidic front, while the reduction of water at the cathodes produces an alkaline front. The H+ and OH− species thus formed not only contribute to electromigration but can also allow changes in soil pH with possible repercussions on soil chemistry. Among the resulting chemical reactions, the dissolution or precipitation of salts and minerals can either facilitate or hinder the release of pollutants fixed in the soil [12].
2. Evolution of the Technology and Its Applications
Although the first investigations on electroosmotic flow date back to the beginning of the 19th century [1][13], the electrokinetic approach was applied only several decades later, initially as a consolidation process for fine soils [14] and subsequently for the recovery of heavy metals [15][16].
In the first investigations [2][9], the removal of contaminants was attempted simply by inducing an electric current through electrodes inserted directly into the contaminated soil, but the efficiency of the remediation was low. Numerous improvements have therefore been proposed, including:
- the optimization of the pH of electrolytic solutions [17];
- the use of ion-exchange membranes to prevent the migration of protons (acidity) and hydroxyl ions (alkalinity) from the electrode wells to the soil [17][18][19];
- the increase in the mobility of pollutants by adding complexing agents [20] and surfactants [21];
- the optimization of the effective volume, by varying the disposition of the electrodes according to the nature of the site and the target contaminants [22][23].
Since electromigration generally provides a greater impact than electroosmosis, for many years EK remediation has mainly focused on charged species such as heavy metals [24][25][26]. More recently, research has focused on the use of EKR technology for the removal of dangerous organic substances from soil [27] or from marine and river sediments [28]. A plethora of technologies have been reported, ranging from simple electrokinetic soil flushing for soils with low hydraulic conductivity, to the use of permeable reactive barriers loaded with granular activated carbon (GAC) [29], zero-valent iron (ZVI) [30][31][32], or even microorganisms [33] used to retain or transform the organic species mobilized by the applied electric fields.[3][4][5][6][7][8][10][11][18][19]
The main challenge of EK technology is the conversion of low solubility pollutants into mobile forms in order to extract them. Therefore, enhancing agents, added to the process fluids, are necessary to obtain an effective removal of all type of contaminants. During an electrokinetic soil flushing, surfactants are normally introduced into the process fluid to allow the formation of micelles (charged particles) with the species target of the remediation. These micelles are then transported across the soil under the effect of the electric field applied to promote the removal of organic and inorganic compounds [2][7], causing electrophoresis to significantly contribute to the remediation process of relatively permeable soils.
The ever-increasing attention to environmental sustainability is now catalyzing the interest in new (bio)remediation technologies, which involves a minimum use of chemicals (and external energy) and consequently implies a lower environmental impact [34]. In this context, electro-bioremediation technologies have recently been investigated, especially following the discovery that many microorganisms are capable of degrading environmental contaminants, including polycyclic aromatic hydrocarbons (PAHs), using electrodes as electron acceptors [35][36][37].
Furthermore, bioelectrochemical systems allow manipulating the redox potential of the contaminated matrix, thus establishing in situ conditions that favor the biodegradation of contaminants [38][39].
On the other hand, the coupling of the electrokinetic approach with bio-remediation and phyto-remediation could represent a more sustainable approach. Their energy requirement is low, the addition of chemicals is often not necessary, and the physicochemical and ‘biological’ (e.g., the fertility) characteristics of the soil at the end of the treatment are improved compared to the initial situation. EK treatment can increase the bioavailability of organic pollutants by facilitating contact between microbes and nutrients and/or pollutants, and the weak electric current may also directly stimulate microbial activity [40][41] or degrade some of the pollutants through an electrolytic reaction [42]. In addition, the application of an EK treatment can improve the growth and respiration of plants (which in turn can facilitate the removal of metals) and facilitate the spread of microorganisms of the rhizosphere (with possible enhancement of the biodegradation of organic contaminants) [43]. Other authors instead argue that the benefits are linked to the influence of the electric field on enzymatic reactions, water activity, and membrane transport [44].
It has been reported that the elimination of organic and/or inorganic contaminants through the combined use of plants and an electric field applied through the soil to be treated is an effective approach [45], able to control the transport of contaminants in the rhizosphere, as well as to prevent the establishment of strong acid or alkaline fronts in the soil [46]. However, suitable operating conditions must be selected to ensure the survival and development of microorganisms and/or plants [47]. Extreme pH values and high temperatures can be produced during the process, which are two of the most critical parameters for keeping microorganisms active [10][48]. Since most processes induced by the electric field have a negative effect on the viability of microorganisms, the simultaneous optimization of both electrokinetic and biological processes can be very challenging [39]. The application of an electric field can also cause negative effects to plants: for example, growth inhibition and death of plants located near the electrodes have been reported [49]. The negative effects of electricity would be linked to changes in soil pH associated with water electrolysis and phytotoxicity due to the increasing bioavailability of metals.
Another recently explored approach is the coupling of EKRT and nanotechnology. Nanomaterials and nanoparticles have peculiar properties which, if properly exploited, can allow first-rate performance while maintaining high sustainability [50][51].
It has been suggested to stabilize or improve the physical properties of a collapsible soil (e.g., loess) by employing the EK technique for the distribution of additives such as nano-silica or lime [52]. On the other hand, the approach can also be applied to achieve remediation goals; for example, the potential of a combined nZVI-EK bioremediation approach (nZVI stands for zero-valent nanoscale iron) to clean up highly polluted aquifers from chlorinated ethenes has been recently explored [53]. It was shown that EK improves the long-term reactivity of nZVI by also stimulating microbial degradation activity by increasing the groundwater temperature.
This entry is adapted from the peer-reviewed paper 10.3390/su13020770
References
- Reuss, F.F. Notice sur un nouvel effet de l’électricité galvanique. Mem. Soc. Imp. Nat. Moscou 1809, 2, 327–337.
- Acar, Y.B.; Alshawabkeh, A.N. Principles of electrokinetic remediation. Environ. Sci. Technol. 1993, 27, 2638–2647.
- Risco, C.; Rodrigo, S.; López-Vizcaíno, R.; Yustres, A.; Sáez, C.; Cañizares, P.; Navarro, V.; Rodrigo, M.A. Electrochemically assisted fences for the electroremediation of soils polluted with 2,4-D: A case study in a pilot plant. Sep. Purif. Technol. 2015, 156, 234–241.
- Schnarr, M.; Truax, C.; Farquhar, G.; Hood, E.; Gonullu, T.; Stickney, B. Laboratory and controlled field experiments using potassium permanganate to remediate trichloroethylene and perchloroethylene DNAPLs in porous media. J. Contam. Hydrol. 1998, 29, 205–224.
- Reddy, K.R.; Saichek, R.E. Effect of soil type on electrokinetic removal of phenanthrene using surfactants and cosolvents. J. Environ. Eng. 2003, 129, 336–346.
- Reddy, K.R.; Danda, S.; Saichek, R.E. Complicating factors of using ethylenediamine tetraacetic acid to enhance electrokinetic remediation of multiple heavy metals in clayey soils. J. Environ. Eng. 2004, 130, 1357–1366.
- McBratney, A.; Field, D.J.; Koch, A. The dimensions of soil security. Geoderma 2014, 213, 203–213.
- Vocciante, M.; Bagatin, R.; Ferro, S. Enhancements in electrokinetic remediation technology: Focus on water management and wastewater recovery. Chem. Eng. J. 2017, 309, 708–716.
- Acar, Y.B.; Gale, R.J.; Alshawabkeh, A.N.; Marks, R.E.; Puppala, S.; Bricka, M.; Parker, R. Electrokinetic remediation: Basics and technology status. J. Hazard. Mater. 1995, 40, 117–137.
- Gill, R.T.; Harbottle, M.J.; Smith, J.W.N.; Thornton, S.F. Electrokinetic-enhanced bioremediation of organic contaminants: A review of processes and environmental applications. Chemosphere 2014, 107, 31–42.
- Reddy, K.R.; Cameselle, C. Electrochemical Remediation Technologies for Polluted Soils, Sediments and Groundwater; John Wiley & Sons: Hoboken, NJ, USA, 2009.
- Rocha, I.M.V.; Silva, K.N.O.; Silva, D.R.; Martínez-Huitle, C.A.; dos Santos, E.V. Coupling electrokinetic remediation with phytoremediation for depolluting soil with petroleum and the use of electrochemical technologies for treating the effluent generated. Sep. Purif. Technol. 2019, 208, 194–200.
- Porrett, R., Jr. Curious galvanic experiments. Ann. Philos. 1816, 8, 74–76.
- Casagrande, L. Electro-osmosis in soils. Géotechnique 1949, 1, 159–177.
- Hamnett, R. A study of the Processes Involved in the Electro-Reclamation of Contaminated Soils. Master’s Thesis, University of Manchester, Manchester, UK, 1980.
- Segall, B.A.; Matthias, J.A.; O’Bannon, C.E. Electro-osmosis chemistry and water quality. J. Geotech. Eng. Div. 1980, 106, 1148–1152.
- Puppala, S.K.; Alshawabkeh, A.N.; Acar, Y.B.; Gale, R.J.; Bricka, M. Enhanced electrokinetic remediation of high sorption capacity soil. J. Hazard. Mater. 1997, 55, 203–220.
- Ottosen, L.M.; Hansen, H.K.; Laursen, S.; Villumsen, A. Electrodialytic remediation of soil polluted with copper from wood preservation industry. Environ. Sci. Technol. 1997, 31, 1711–1715.
- Li, Z.; Yu, J.W.; Neretnieks, I. Electroremediation: Removal of heavy metals from soils by using cation selective membrane. Environ. Sci. Technol. 1998, 32, 394–397.
- Wong, J.S.; Hicks, R.E.; Probstein, R.F. EDTA-enhanced electroremediation of metal-contaminated soils. J. Hazard. Mater. 1997, 55, 61–79.
- Ko, S.O.; Schlautman, M.A.; Carraway, E.R. Cyclodextrin-enhanced electrokinetic removal of phenanthrene from a model clay soil. Environ. Sci. Technol. 2000, 34, 1535–1541.
- Alshawabkeh, A.N.; Yeung, A.T.; Bricka, M.R. Practical aspects of in-situ electrokinetic extraction. J. Environ. Eng. 1999, 125, 27–35.
- Alshawabkeh, A.N.; Gale, R.J.; Ozsu-Acar, E.; Bricka, M.R. Optimization of 2-D electrode configuration for electrokinetic remediation. J. Soil Contam. 1999, 8, 617–635.
- Kim, K.-J.; Kim, D.-H.; Yoo, J.-C.; Baek, K. Electrokinetic extraction of heavy metals from dredged marine sediment. Sep. Purif. Technol. 2011, 79, 164–169.
- Rozas, F.; Castellote, M. Electrokinetic remediation of dredged sediments polluted with heavy metals with different enhancing electrolytes. Electrochim. Acta 2012, 86, 102–109.
- Iannelli, R.; Masi, M.; Ceccarini, A.; Ostuni, M.B.; Lageman, R.; Muntoni, A.; Pomi, R. Electrokinetic remediation of metal-polluted marine sediments: Experimental investigation for plant design. Electrochim. Acta 2015, 181, 146–159.
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186, 24–41.
- Mukimin, A.; Zen, N.; Purwanto, A.; Wicaksono, K.A.; Vistanty, H.; Alfauzi, A.S. Application of a full-scale electrocatalytic reactor as real batik printing wastewater treatment by indirect oxidation process. J. Environ. Chem. Eng. 2017, 5, 5222–5232.
- Munoz-Morales, M.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. A new strategy for the electrolytic removal of organics based on adsorption onto granular activated carbon. Electrochem. Commun. 2018, 90, 47–50.
- Warner, S.D.; Ferro, S.; De Battisti, A. Barriere permeabili reattive—Considerazioni generali e stato dell’arte. La Chimica e l’Industria (Milan Italy) 2006, 88, 44–46.
- Araújo, R.; Castro, A.C.M.; Baptista, J.S.; Fiúza, A. Nanosized iron based permeable reactive barriers for nitrate removal–Systematic review. Phys. Chem. Earth 2016, 94, 29–34.
- Naje, A.S.; Chelliapan, S.; Zakaria, Z.; Ajeel, M.A.; Alaba, P.A. A review of electrocoagulation technology for the treatment of textile wastewater. Rev. Chem. Eng. 2017, 33, 263–292.
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227.
- Cappello, S.; Cruz Viggi, C.; Yakimov, M.; Rossetti, S.; Matturro, B.; Molina, L.; Segura, A.; Marqués, S.; Yuste, L.; Sevilla, E.; et al. Combining electrokinetic transport and bioremediation for enhanced removal of crude oil from contaminated marine sediments: Results of a long-term, mesocosm-scale experiment. Water Res. 2019, 157, 381–395.
- Aulenta, F.; Canosa, A.; Reale, P.; Rossetti, S.; Panero, S.; Majone, M. Microbial reductive dechlorination of trichloroethene to ethene with electrodes serving as electron donors without the external addition of redox mediators. Biotechnol. Bioeng. 2009, 103, 85–91.
- Wang, H.; Luo, H.; Fallgren, P.H.; Jin, S.; Ren, Z.J. Bioelectrochemical system platform for sustainable environmental remediation and energy generation. Biotechnol. Adv. 2015, 33, 317–334.
- Domínguez-Garay, A.; Quejigo, J.R.; Dörfler, U.; Schroll, R.; Esteve-Núñez, A. Bioelectroventing: An electrochemical-assisted bioremediation strategy for cleaning-up atrazine-polluted soils. Microb. Biotechnol. 2018, 11, 50–62.
- Yan, F.; Chen, W.; Reible, D. Electrochemical stimulation of PAH biodegradation in sediment. Soil Sediment Contam. 2015, 24, 143–156.
- Barba, S.; López-Vizcaíno, R.; Saez, C.; Villaseñor, J.; Cañizares, P.; Navarro, V.; Rodrigo, M.A. Electro-bioremediation at the prototype scale: What it should be learned for the scale-up. Chem. Eng. J. 2018, 334, 2030–2038.
- Kim, S.H.; Han, H.Y.; Lee, Y.J.; Kim, C.W.; Yang, J.W. Effect of electrokinetic remediation on indigenous microbial activity and community within diesel contaminated soil. Sci. Total Environ. 2010, 408, 3162–3168.
- Velasco-Alvarez, N.; González, I.; Damian-Matsumura, P.; Gutiérrez-Rojas, M. Enhanced hexadecane degradation and low biomass production by Aspergillus niger exposed to an electric current in a model system. Biores. Technol. 2011, 102, 1509–1515.
- Aćimović, D.D.; Karić, S.D.; Nikolić, Ž.M.; Brdarić, T.P.; Tasić, G.S.; Kaninski, M.P.M.; Nikolić, V.M. Electrochemical oxidation of the polycyclic aromatic hydrocarbons in polluted concrete of the residential buildings. Environ. Pollut. 2017, 220, 393–399.
- Huang, H.; Tang, J.; Niu, Z.; Giesy, J.P. Interactions between electrokinetics and rhizoremediation on the remediation of crude oil-contaminated soil. Chemosphere 2019, 229, 418–425.
- Bi, R.; Schlaak, M.; Siefert, E.; Lord, R.; Connolly, H. Influence of electrical fields (AC and DC) on phytoremediation of metal polluted soils with rapeseed (Brassica napus) and tobacco (Nicotiana tabacum). Chemosphere 2011, 83, 318–326.
- Denvir, A.; Hodko, D.; Van Hyfte, J.; Magnuson, J.W. Methods for Enhancing Phytoextraction of Contaminants from Porous Media Using Electrokinetic Phenomena. U.S. Patent No. 6,145,244, 14 November 2000.
- Aboughalma, H.; Bi, R.; Schlaak, M. Electrokinetic enhancement on phytoremediation in Zn, Pb, Cu and Cd contaminated soil using potato plants. J. Environ. Sci. Health A 2008, 43, 926–933.
- Cameselle, C.; Gouveia, S. Electrokinetic remediation for the removal of organic contaminants in soils. Curr. Opin. Electrochem. 2018, 11, 41–47.
- Yeung, A.T.; Gu, Y.Y. A review on techniques to enhance electrochemical remediation of contaminated soils. J. Hazard. Mater. 2011, 195, 11–29.
- O’Connor, C.S.; Lepp, N.W.; Edwards, R.; Sunderland, G. The combined use of electrokinetic remediation and phytoremediation to decontaminate metal-polluted soils: A laboratory-scale feasibility study. Environ. Monit. Assess. 2003, 84, 141–158.
- Reverberi, A.P.; Vocciante, M.; Salerno, M.; Ferretti, M.; Fabiano, B. Green synthesis of silver nanoparticles by low-energy wet bead milling of metal spheres. Materials 2020, 13, 63.
- Trofa, M.; D’Avino, G.; Fabiano, B.; Vocciante, M. Nanoparticles synthesis in wet-operating stirred media: Investigation on the grinding efficiency. Materials 2020, 13, 4281.
- Hosseini, A.; Haeri, S.M.; Mahvelati, S.; Fathi, A. Feasibility of using electrokinetics and nanomaterials to stabilize and improve collapsible soils. J. Rock Mech. Geotech. 2020, 11, 1055–1065.
- Czinnerová, M.; Vološčuková, O.; Marková, K.; Ševců, A.; Černík, M.; Nosek, J. Combining nanoscale zero-valent iron with electrokinetic treatment for remediation of chlorinated ethenes and promoting biodegradation: A long-term field study. Water Res. 2020, 175, 115692.