Carbon Dots (CDs) are a kind of 0-D emissive spheroidal carbon-based nanostructures with a size smaller than 20 nm. The CDs, in fact, stand in between organic (polymers) and inorganic materials (black carbon), macromolecules, and nanoparticle, between bottom-up (polycyclic aromatic compounds) and top-down synthesis (laser ablation of graphene, etc.).
- Carbon Dot
- Citric Acid
- Photoluminescence
1. Introduction
Carbon dots (CDs) are highly-emissive nanoparticles obtained through fast and cheap syntheses. The understanding of CDs' luminescence, however, is still far from being comprehensive. The intense photoluminescence can have different origins: molecular mechanisms, oxidation of polyaromatic graphene-like layers, and core–shell interactions of carbonaceous nanoparticles.[1][2][3] The citric acid (CA) is one of the most common precursors for CD preparation because of its high biocompatibility.[4]
2. Optical Properties of CA-Based CDs
The striking possibility of synthesizing such bright fluorescent nanoparticles using simple protocols and cheap precursors is the reason for the broad interest in CDs.[5] However, everything comes at a cost. The multiple carbonization reactions which drive the formation of the emitting nanoparticles are largely uncontrolled, and it appears to be difficult to disentangle such a complex network of processes occurring during the CD formation. Moreover, when hydrothermal, microwave treatment, or thermal degradation are used to synthesize CDs, the chemical reactions which lead to the formation of the carbon nanoparticles are very different from those which typically occur in organic chemistry. A careful retro-engineering process is, therefore, at the basis of real CDs nanotechnology. Bottom-up routes are effective methods to obtain high quality CDs with some organics as precursors. CDs can be synthesized from CA via hydrothermal or solvothermal methods, microwave treatments, thermal decomposition, etc.
N-source precursors allow modulating the photophysical properties of CDs in a wide range of emissions. As shown in Table 2, the emission from CA-based CDs can be tuned from the blue to the red with remarkable QYs (>90%). The QY, however, gradually reduces when the characteristic emission is shifted towards higher wavelengths. According to the reports in Table 1, the CA-based CDs can also exhibit near-infrared photoluminescence, especially when synthesized in aprotic polar solvents, such as DMF and formamide.
Table 1. The preparation and optical properties of some CA-based CDs. DMF: dimethylformamide.
|
Methods |
Precursors (Including CA) |
Emission |
QYs |
Ref. |
|
Microwave treatment (700 W, 40 s, aqueous solution) |
L-cysteine |
425 nm |
78% |
[6] |
|
Hydrothermal method (180 °C, 8 h) |
Diethylenetriamine |
433 nm |
98% |
[7] |
|
Hydrothermal method (200 °C, 5 h) |
Ethylenediamine |
445 nm |
80.6% |
[8] |
|
Microwave treatment (400 W, 80 min, 150 °C, aqueous solution) |
Ethylenediamine |
445 nm |
83% |
[9] |
|
Hydrothermal method (160 °C, 4 h) |
Ethylenediamine |
450 nm |
94% |
[10] |
|
Microwave treatment (800 W, 4 min, aqueous solution) |
L-cysteine, dextrin |
495 nm |
22% |
[11] |
|
Hydrothermal method (190 °C, 2 h) |
Urea |
455 nm 510 nm |
29% 30% |
[12] |
|
Thermal decomposition (170 °C, 70 min) |
Dicyandiamide |
528 nm |
73.12% |
[13] |
|
Hydrothermal method (200 °C, 12 h) |
Urea, ZnCl2 |
580 nm |
51.2% |
[14] |
|
Solvothermal method (180 °C, 4 h, formamide solution) |
Ethanediamine |
627 nm |
53% |
[15] |
|
Microwave treatment (400 W, formamide solution) |
- |
640 nm |
22.9% |
[16] |
|
Solvothermal method (160 °C, 6 h, DMF solution) |
Urea |
760 nm |
10% |
[17] |
2.1. Multicolor Photoluminescence
Some specific syntheses allow preparing CA-based CDs with multicolor tunable photoluminescence. This means that minor variations of the synthesis conditions lead to different emissions. By controlling the solvothermal conditions, Miao et al.[18] prepared multiple emissive CDs. The shift of emission from the blue to the red of the visible spectrum is related to the presence of the carboxylic groups and the increasing graphite content in the CDs. Hola and his co-workers[19] prepared multicolor emissive CDs via solvothermal treatment of CA and urea in a formamide solution. The obtained products were further purified and separated by column chromatography into four fractions showing emissions from blue to red. Herein, however, the redshift of the photoluminescence was attributed to increasing graphitic nitrogen contents, as also confirmed by time-dependent density functional theory (TD-DFT). Interestingly, all the works focused on the multicolor photoluminescence of CDs agree in considering the graphitic structure (derived from the carbonization) as a key factor to control the CDs' multicolor emission.
While the luminescence appears to be largely controlled by the degree of graphitization of the CD structure, the mechanism controlling the fluorescence is still unclear. This property seems to be affected by many other factors which induce a multicolor fluorescence. Zhu et al.[20], for example, proved that the introduction of metal ions into CA-based CDs structure prepared via magnetic hyperthermia approach leads to a multicolor-fluorescence over a wide range of the visible spectrum. Moreover, some CDs exhibit a long fluorescence lifetime, such as long afterglow performance. CDs fabricated via hydrothermal treatment of CA, acrylamide, and urea exhibit room-temperature phosphorescence with a long lifetime reaching up to 459 ms.[21] Boron is also an important doping agent in the development of CDs featuring ultralong lifetime room temperature phosphorescence. Boron-doped CDs exhibit bright yellow-green afterglow with a remarkable QY of 23.5% and a lifetime of 1.17 s.[22]
2.2. Surface Modifications
Surface modification is, in general, an effective strategy to enhance CDs' optical performances. Usually, the CDs obtained by pyrolysis of CA show a weak fluorescence with a short lifetime. In our previous work,[4] it was found that the surface functionalization with 3-aminopropyltriethoxysilane (APTES) reduces the CDs fluorescence quenching due to the formation of a passivation layer. The 3-glycidyloxypropyltrimethoxysilane (GPTMS) is also a possible alternative to modify the surface via an epoxy–amine reaction.[23] In addition to the native luminescence at 430 nm, the GPTMS-grafted CDs show a new emission at 490 nm due to the polyethylene oxide species.
Liu et al.[24] prepared highly emissive solid-state CDs via one-step hydrothermal method with CA as the carbon source and branched poly(ethylenimine) (b-PEI) as the surface passivation agent. The introduction of b-PEI chains can prevent the collisions of CD emissive centers, which further avoids the aggregation-induced quenching.
2.3. External Variables Controlling the Emission
The optical response of phosphors, including CDs, usually are temperature dependent. Kalytchuk et al.[25] prepared nitrogen/sulfur-co-doped CDs by hydrothermal treatment of CA and L-cysteine, which show temperature-dependent absorption, steady-state and transient photoluminescence, and lifetime.
When CDs are used in solution, pH is also a pivotal parameter capable of tuning the emission. CDs made of CA and polyethylenimine (PEI) or 2,3-diaminopyridine (DAP) have shown a reduction of the QYs from ≈ 40–50% to less than 9% when dissolved in an aqueous solution of pH ≈1 and to 21% when the pH has been increased to 12.[26]
Concentration-dependent emission is another feature of CDs, which is usually compared with aggregation-induced emission. Wang et al.[27] synthesized a type of concentration-dependent fluorescent CD by microwave treatment of CA and ammonia. The photoluminescence shows a remarkable red-shift when the CDs' concentration increases from 0.78 to 10.42 mg mL−1 in an aqueous solution.
3. Optical Applications
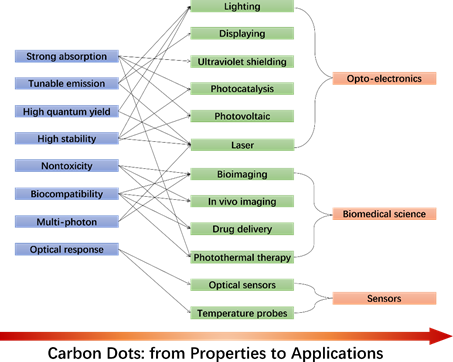
The excellent optical properties make CDs a promising material to be used in many potential applications, as resumed in Figure 1. The strong absorption endowed by the CDs could be efficiently applied to ultraviolet shielding devices.[28] It is also widely recognized that CDs boost the photocatalytic reactions. CD-based photocatalysts also work well in the near-infrared region where up-conversion is observed.[29] CDs-based devices are expected to replace toxic compounds or rare elements in monitors and fluorescent bulbs even if, at the moment, they lack the efficiency requested in the red region. The state of the art for CA-based CDs is multicolor emission with QYs exceeding 50% in the red.[15][6]
The surface functionalization has already proved to further enhance the CD's optical properties. Organosilane-linked CDs, for instance, show both down and up-conversion photoluminescence and exhibit significant multi-photon absorption at room temperature.[30] Although multiphoton excited fluorescence is an emerging research topic, however, it is clear that specifically designed CDs could provide dramatic advances in cutting-edge optical applications such as infrared light detectors, microcavity lasers, etc.
CDs are sensitive to environment changes, such as pH, ions strength, and temperature, which set the conditions for CDs-based optical sensors. For example, CDs with hydroxyl and carboxyl groups on their surface have shown to work well as pH sensors. Currently, CDs have been extensively used to detect iron (III) due to a strong coordination interaction between Fe3+ and the phenolic groups of CDs. Finally, the nontoxicity and the biocompatibility make CDs ideal nanomaterials to develop innovative bioimaging techniques, biosensors, drug-delivery carriers, and nano-based antioxidant formulations.
In the future, new and effective technologies are required for low-cost production of high-quantity CDs.
Figure 1. CDs' optical properties and potential applications.
4. Summary and Outlook
Although the large variability in chemical composition and structure of the different CDs, the large number of works published so far allows identifying some general trends and common phenomena in the preparation and optical performances of the CDs.
The carbonization temperature is a key factor for controlling the growth of the carbon core and the density of surface groups in the CDs systems. A carbon core is usually obtained at high temperature, while the surface groups reduce as a function of the temperatures. An intermediate temperature (150-200 °C for CA-based CDs), therefore, should be considered to maximize the QY.
Reaction time plays a role similar to the reaction temperature in the CDs' synthesis. Extending the carbonization for longer times enhances the degree of carbonization and reduces the functional groups on the surface. In the quest of CDs with high quantum yields, it is therefore of paramount importance the optimization of the reaction time to achieve a compromise between the core formation and the chemical composition of the surface. When a solvothermal approach is used, the choice of the solvent affects the CDs fluorescence. Generally speaking, the solvents can also serve as Nitrogen sources towards doping of the CDs' structure.
CA-based CDs show multicolor fluorescence. However, the maximum of the emission is shifted towards longer wavelengths when organic, apolar, and N-containing solvents are used instead of water during the solvothermal synthesis. The nitrogen-doping dramatically contributes to enhancing the QY. This effect, however, depends on the chemical composition of the reagents used for doping. It has been found that, in general, primary amines allows increasing the QY more than secondary and tertiary amines NH2- > -NH- > N≡.
Although the machinery of CD fluorescence is still mostly unexplored, it seems that both amorphous and graphitic carbon atoms could contribute to this effect. The size of the carbon core also influences the emission, due to the quantum confinement effect, by red shifting the fluorescence redshift.
Concerning the technological advancements, although the CDs show a set of promising features, full optimization of the photophysical properties is required to promote the development of innovative functional devices.
This entry is adapted from the peer-reviewed paper 10.3390/c7010002
References
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355.
- Malfatti, L.; Innocenzi, P. Sol-gel chemistry for carbon dots. Chem. Rec. 2018, 18, 1192.
- Ren, J.; Stagi, L.; Innocenzi, P. Fluorescent carbon dots in solid-state: From nanostructures to functional devices. Prog. Solid State Chem. 2020, 100295.
- Ludmerczki, R.; Mura, S.; Carbonaro, C.M.; Mandity, I.M.; Carraro, M.; Senes, N.; Garroni, S.; Granozzi, G.; Calvillo, L.; Marras, S. Carbon dots from citric acid and its intermediates formed by thermal decomposition. Chem. Eur. J. 2019, 25, 11963.
- Ren, J.; Malfatti, L.; Innocenzi, P. Citric acid derived carbon dots, the challenge of understanding the synthesis-structure relationship. C 2021, 7, 2.
- Anjana, R.; Devi, J.A.; Jayasree, M.; Aparna, R.; Aswathy, B.; Praveen, G.; Lekha, G.; Sony, G. S, N-doped carbon dots as a fluorescent probe for bilirubin. Microchim. Acta 2018, 185, 11.
- Zhang, W.; Shi, L.; Liu, Y.; Meng, X.; Xu, H.; Xu, Y.; Liu, B.; Fang, X.; Li, H.; Ding, T. Supramolecular interactions via hydrogen bonding contributing to citric-acid derived carbon dots with high quantum yield and sensitive photoluminescence. RSC Adv. 2017, 7, 20345.
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953.
- Xiao, Q.; Liang, Y.; Zhu, F.; Lu, S.; Huang, S. Microwave-assisted one-pot synthesis of highly luminescent N-doped carbon dots for cellular imaging and multi-ion probing. Microchim. Acta 2017, 184, 2429.
- Qu, D.; Zheng, M.; Zhang, L.; Zhao, H.; Xie, Z.; Jing, X.; Haddad, R.E.; Fan, H.; Sun, Z. Formation mechanism and optimization of highly luminescent N-doped graphene quantum dots. Sci. Rep. 2014, 4, 5294.
- Liu, Q.; Zhang, N.; Shi, H.; Ji, W.; Guo, X.; Yuan, W.; Hu, Q. One-step microwave synthesis of carbon dots for highly sensitive and selective detection of copper ions in aqueous solution. New J. Chem. 2018, 42, 3097
- Mura, S.; Ludmerczki, R.; Stagi, L.; Garroni, S.; Carbonaro, C.M.; Ricci, P.C.; Casula, M.F.; Malfatti, L.; Innocenzi, P. Integrating sol-gel and carbon dots chemistry for the fabrication of fluorescent hybrid organic-inorganic films. Sci. Rep. 2020, 10, 4770.
- Hou, J.; Wang, W.; Zhou, T.; Wang, B.; Li, H.; Ding, L. Synthesis and formation mechanistic investigation of nitrogen-doped carbon dots with high quantum yields and yellowish-green fluorescence. Nanoscale 2016, 8, 11185.
- Cheng, J.; Wang, C.; Zhang, Y.; Yang, S.; Chen, S. Zinc ion-doped carbon dots with strong yellow photoluminescence. RSC Adv. 2016, 6, 37189.
- Ding, H.; Wei, J.; Zhong, N.; Gao, Q.; Xiong, H. Highly efficient red-emitting carbon dots with gram-scale yield for bioimaging. Langmuir 2017, 33, 12635.
- Sun, S.; Zhang, L.; Jiang, K.; Wu, A.; Lin, H. Toward high-efficient red emissive carbon dots: Facile preparation, unique properties, and applications as multifunctional theranostic agents. Chem. Mater. 2016, 28, 8659.
- Li, D.; Jing, P.; Sun, L.; An, Y.; Shan, X.; Lu, X.; Zhou, D.; Han, D.; Shen, D.; Zhai, Y. Near-infrared excitation/emission and multiphoton-induced fluorescence of carbon dots. Adv. Mater. 2018, 30, 1705913.
- Miao, X.; Qu, D.; Yang, D.; Nie, B.; Zhao, Y.; Fan, H.; Sun, Z. Synthesis of carbon dots with multiple color emission by controlled graphitization and surface functionalization. Adv. Mater. 2018, 30, 1704740.
- Hola, K.; Sudolska, M.; Kalytchuk, S.; Nachtigallova, D.; Rogach, A.L.; Otyepka, M.; Zboril, R. Graphitic nitrogen triggers red fluorescence in carbon dots. ACS Nano 2017, 11, 12402.
- Zhu, Z.; Cheng, R.; Ling, L.; Li, Q.; Chen, S. Rapid and large-scale production of multi-fluorescence carbon dots via magnetic hyperthermia method. Angew. Chem. Int. Ed. 2019, 59, 3099.
- Li, H.; Ye, S.; Guo, J.; Kong, J.; Song, J.; Kang, Z. The design of room-temperature-phosphorescent carbon dots and their application as a security ink. J. Mater. Chem. 2019, 7, 10605.
- Xie, Z.; Zheng, M. Colour-tunable ultralong-lifetime room temperature phosphorescence with external heavy-atom effect in boron-doped carbon dots. Chem. Eng. J. 2020, 127647.
- Suzuki, K.; Malfatti, L.; Takahashi, M.; Carboni, D.; Messina, F.; Tokudome, Y.; Takemoto, M.; Innocenzi, P. Design of carbon dots photoluminescence through organo-functional silane grafting for solid-state emitting devices. Sci. Rep. 2017, 7, 5469.
- Liu, E.; Li, D.; Zhou, X.; Zhou, G.; Xiao, H.; Zhou, D.; Tian, P.; Guo, R.; Qu, S. Highly emissive carbon dots in solid state and their applications in light-emitting devices and visible light communication. ACS Sustain. Chem. Eng. 2019, 7, 9301.
- Kalytchuk, S.; Poláková, K.I.; Wang, Y.; Froning, J.P.; Cepe, K.; Rogach, A.L.; Zboˇril, R. Carbon dot nanothermometry: Intracellular photoluminescence lifetime thermal sensing. ACS Nano 2017, 11, 1432.
- Meierhofer, F.; Dissinger, F.; Weigert, F.; Jungclaus, J.; Müller-Caspary, K.; Waldvogel, S.R.; Resch-Genger, U.; Voss, T. Citric acid based carbon dots with amine type stabilizers: pH-specific luminescence and quantum yield characteristics. J. Phys. Chem. 2020, 124, 8894
- Wang, C.; Hu, T.; Wen, Z.; Zhou, J.; Wang, X.; Wu, Q.; Wang, C. Concentration-dependent color tunability of nitrogen-doped carbon dots and their application for iron (III) detection and multicolor bioimaging. J. Colloid Interface Sci. 2018, 521, 33.
- Xie, Z.; Du, Q.; Wu, Y.; Hao, X.; Liu, C. Full-band UV shielding and highly daylight luminescent silane-functionalized graphene quantum dot nanofluids and their arbitrary polymerized hybrid gel glasses. J. Mater. Chem. 2016, 4, 9879.
- Zhang, Y.; Park, M.; Kim, H.Y.; Ding, B.; Park, S.-J. A facile ultrasonic-assisted fabrication of nitrogen-doped carbon dots/BiOBr up-conversion nanocomposites for visible light photocatalytic enhancements. Sci. Rep. 2017, 7, 45086.
- Zhang, W.; Ni, Y.; Xu, X.; Lu, W.; Ren, P.; Yan, P.; Siu, C.K.; Ruan, S.; Yu, S.F. Realization of multiphoton lasing from carbon nanodot microcavities. Nanoscale 2017, 9, 5957.