Reactive organophosphates (OPs) comprise of collectively a group of phosphorous-based toxic chemicals that cause life-threatening toxic symptoms in humans. These include nerve agents and agricultural pesticides. Nanomaterial applications offer a high potential in developing nanosensors for sensitive OP detection and quantitative analysis.
- reactive organophosphate
- oxime
- nanomaterials
- sensors
- electrochemistry
- luminescence
1. Introduction
The term reactive organophosphates (OPs) refers collectively to a group of phosphorous-based toxic chemicals that cause life-threatening toxic symptoms in humans. These comprise OP nerve agents such as sarin, soman and VX, as well as OP-based pesticides like chlorpyrifos, paraoxon and malaoxon, among others (Figure 1) [1][2][3]. Despite their much lower toxicity, the OP pesticides are still formidable due to their wide distribution ranging from insect controls in the agricultural and horticultural sectors to pest treatments for domestic pets, farm animals and houses[4]. OP toxicity is commonly attributed to a phosphorous (thio)ester core that serves as its reactive functionality. It is highly susceptible to engaging in a covalent conjugation with a nucleophilic residue present in proteins and cellular enzymes in plasmas[1][5][6][7][8]. This OP reaction results in a covalent protein modification and thus loss of their original activity.
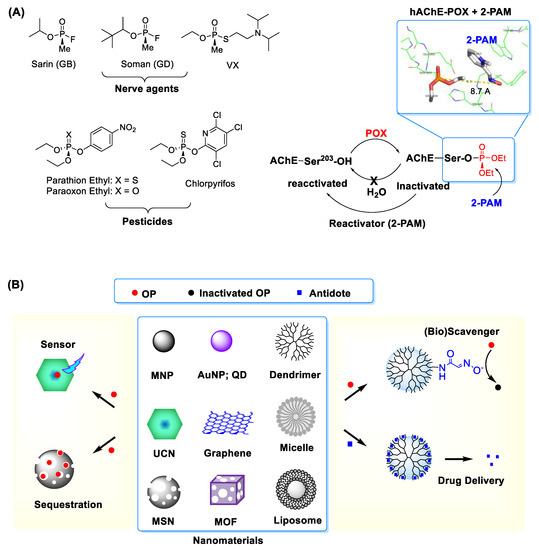
Figure 1. (A) (left) Types of representative organophosphates (OP) including nerve agents and pesticides and (right) covalent inhibition of acetylcholine esterase (AChE) by OP such as paraoxon ethyl (POX). Inset: An X-ray crystal structure for POX-inactivated human AChE in complex with 2-PAM, an enzyme reactivator which works by nucleophilic dephosphorylation (protein data bank (PDB) code 5HFA ref [9]). Adapted with permission from ref [10], Copyright 2019, The Royal Society of Chemistry. (B) Selected nanomaterials and their applications in OP detection and treatment. Abbreviations: MNP = magnetic nanoparticle, AuNP = gold (Au) NP, QD = quantum dot, MSN = mesoporous silica nanoparticle, MOF = metal-organic framework, UCN = upconversion nanocrystal.
OP exposures remain a source of serious safety concerns given the history of accidental or terrorist incidents and increasingly indiscriminate use of OP pesticides that is responsible for environmental pollution and crop contamination [11]. In particular, persistent exposures to OP pesticides result in delayed or chronic toxicity in affected organisms[2][3]. Despite such concerns, current capability to address OP issues remains suboptimal due to paucity of advanced technologies that enable for sensitive OP detection or effective treatment. Nanomaterials have made a growing impact on developing therapeutic agents [12][13][14][15][16][17][18][19] and sensors[20] in numerous areas. These are classified as a group of objects or structures in a nanometer size range which display functional properties distinct from bulk materials[21][22]. Such properties are characterized largely by their composition, size and shapes, which include nanospheres[23][24], nanotubes [25], planar nanosheets [26], nanodisks [26][27], nanocages [28] and nanorods [24] (Figure 1). They allow structural modifications and offer functional capabilities through magnetic control, light absorption, fluorescence, luminescence, cavity loading or pore gating. These properties make nanomaterials highly applicable for sensors[28], drug delivery platforms [12]or nanoscale reactors [29][30]. Therefore, nanomaterial applications offer a high potential to address existing OP-related serious problems.
2. Nanosensors for Reactive Organophosphate Detection
2.1. Electrochemistry
2.1.1. AChE-Immobilized Electrode
Electrochemical detection constitutes one of fundamental approaches in biosensor design for OP analysis [31]. This often relies on fabricating an OP-responsive electrode through its surface functionalization such as by immobilization with AChE [31][32][33][34]. This enzyme functionalization is therefore responsible for generating an OP-specific signal in amperometry or voltammetry when its immobilized enzyme loses its catalytic activity upon inactivation by OP [31][32][33]. This detection method is validated for its ability to detect individual OP pesticides or their mixture.
2.1.2. AChE-Immobilized Nanosensor
In an electrochemical nanosensor design, AChE is immobilized on the nanoparticle (NP) surface in lieu of the bulk electrode surface. This approach has been applied to magnetic nanoparticles (MNPs) such as iron oxide (Fe3O4) nanoparticle (IONP) [35] and nano Fe-Ni [36], each offering an important benefit of magnetic control. Thus, using AChE-immobilized MNPs allows temporal and spatial control of MNP localization in an working electrode or screen-printed electrode under an applied magnetic field [35] as reported by Rodrigues et al. In this study, they report unique benefits such as ability for nanosensor assembly on demand and convenience in electrode renewal (cleaning). These are otherwise not available simply by permanent AChE immobilization on the electrode surface. AChE immobilization in MNP-based nanosensors can be achieved by protein crosslinking through glutaraldehyde [35], Ni-histidine tag [37][38] or light responsive polymer [37]. Their sensitivity for OP detection is validated with pesticides such as chlorpyrifos and malathion with limit of detection (LOD) as low as sub nM (Table 1).
Table 1. Nanomaterial-enabled sensors developed for OP analysis.
| Detection | Concept | Design | OP Analyte (LOD) |
|---|---|---|---|
| Electrochemistry | AChE Inhibition | IONP@AChE | Chlorpyrifos oxon, malathion (0.3 nM) |
| nano Fe-Ni@AChE | Phosmet (0.1 nM) | ||
| AuNP-CaCO3@AChE | Malathion, chlorpyrifos (0.1 nM) | ||
| nano Ag@Chitosan-AChE | POX (15 nM) | ||
| MSN@AChE | Dimethoate (6.5 nM) | ||
| Anti-OP Antibody | GNS@Anti-parathion Ab | Parathion (0.2 fM) | |
| OP Adsorption | rGO@Cu | Parathion, fenitrothion, malathion (3 nM) | |
| rGO@AuNP-polymer | Malathion (0.1 nM) | ||
| GNS@AuNP | Parathion methyl (2 nM) | ||
| OP Reaction | GO@AuNP-acetophenone oxime | Diethyl cyanophosphonate, dimethoate, fenitrothion | |
| Fluorescence (Luminescence) Spectroscopy | AChE Inhibition | Cd-Te QD | Paraoxon, GB, VX (0.1–8.0 nM) |
| OP Adsorption | CdTe QD | Chlorpyrifos (0.1 nM) | |
| ZnS-Mn QD | Diethyl phosphorothioate | ||
| Hf-doped MOF | Methylphosphonate | ||
| AuNP@Rhodamine | Ethoprophos (37 nM) | ||
| OP Reaction | CdS QD + Eosin Y | Chlorpyrifos (29 nM) | |
| UCN@Oxime probe | Dimethoate (0.14 μM) | ||
| Colorimetry & Spectrophotometry | AChE Inhibition | AuNR + AChE | Dichlorvos (45 fM) |
| OP Adsorption | AuNP, AgNP | Ethion, parathion | |
| AuNP@Rhodamine | Ethoprophos (37 nM) | ||
| Nano Ag@PVP | Chlorpyrifos (14 nM) |
AuNP = gold nanoparticle; GNS = graphene nanosheet; rGO = reduced graphene oxide; IONP = iron oxide nanoparticle; MNP = magnetic nanoparticle; MOF = metal-organic framework; MSN = mesoporous silica nanoparticle; PVP = polyvinylpyrrolidone; QD = quantum dot; UCN = upconversion nanoparticle.
Non-magnetic NPs are also employed in developing electrochemical nanosensors. These include gold nanoparticle (AuNP) [39], nano Ag [40]and mesoporous silica nanoparticle (MSN) [41], each functionalized by AChE immobilization or non-covalent encapsulation for OP specificity. These nanosensors offer sufficient sensitivity to detect a wide range of OP pesticides as listed in Table 1.
2.1.3. Antibody-Immobilized Nanosensor
Another approach for OP detection involves using an antibody raised against a specific OP species. This is described in a study reported by Mehta et al. [42] in which an anti-parathion antibody was immobilized on the surface of graphene nanosheet (GNS). As a two-dimensional nanostructure, GNS displays an excellent degree of conductance for electrons, which is hence highly suited for application in electrochemical biosensing. This GNS-based immunosensor showed high detection sensitivity for parathion or parathion-like pesticides with LOD as low as fM [42]. However, despite such sensitivity, using an immunosensor has certain drawbacks because its employed antibody is able to recognize only a specific subset of OPs and not applicable to a broader spectrum of OPs [42].
2.1.4. OP-Responsive Nanosensor
As introduced briefly above, GNS display unique features in its structure and property beneficial for electrochemical OP detection. These include high surface area-to-volume ratio, ultralow thickness and high electronic conductance [43]. Their combination confers GNS with sensitive ability to respond to OP adsorption or reaction that occurs on its surface. This is illustrated with a copper-graphene nanocomposite in Figure 2A that shows ability to detect sulfur-containing OP pesticides [44]. Such GNS-based OP detection is further validated using copper-coated reduced graphene oxide (rGO) [44], AuNP-coated rGO [45] and AuNP-coated GNS [46]. Besides, GNS nanosensors are designed by surface modification with an OP-specific probe molecule that engages in selective OP recognition and/or its reaction. Huixiang et al. [47] validated this concept using GO@AuNP functionalized with 4-aminoacetophenone oxime. Thus, an electrode fabricated with this graphene nanocomposite has led to OP detection with LOD at low nM (Table 1).
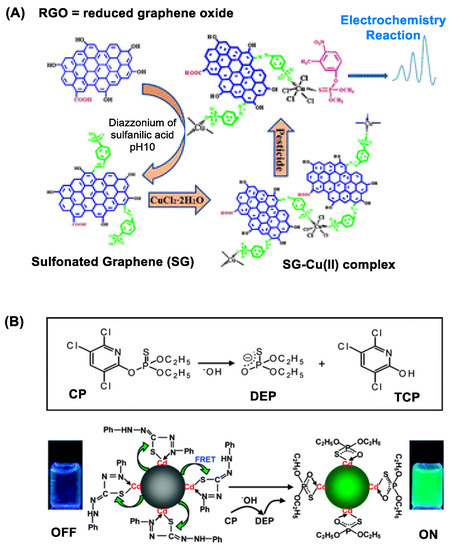
Figure 2. (A) A copper (II)-functionalized graphene nanocomposite applied for sensing sulfur-containing organophosphate (OP) pesticides. Reproduced with permission from [44], Copyright 2013, The Royal Society of Chemistry. (B) Dithizone-coordinated CdTe quantum dot (QD) applied for chlorpyrifos detection. Its concept of detection involves restoration of its fluorescence by dithizone replacement with diethylphosphorothioate, a hydrolytic byproduct of chlorpyrifos. Reproduced with permission from [48], Copyright 2010, American Chemical Society.
In summary, electrochemical nanosensors have shown promising capabilities for OP detection. These are designed with nanomaterials such as IONP [35][37][38], nano Fe-Ni [36], AuNP [39], nano Ag [40], MSN [41] or graphene-based NP [44][45][46][47], each functionalized with AChE [35][36][39][40][41], OP antibody [42] or OP-reactive moiety [47]. These nanosensors offer characteristic advantages including high loading capacity in electrodes, high sensitivity, and fast onset of action due to a narrow spacing between interacting electrodes. Their capabilities are attributable to a combination of their nanometer size, shape and other design features which are not available by conventional bulk electrodes [3][35].
2.2. Absorbance, Fluorescence and Luminescence Spectroscopy
In general, most OPs do not contain chromophores that are applicable for spectroscopic detection by UV–Vis absorbance or fluorescence. There are only few OPs which contain aromatic moieties for UV absorbance such as parathion, paraoxon (POX) and fenitrothion[49], each containing a 4-nitrophenyl group. However, their direct detection by spectrometry is not efficient because their molar absorptivity is practically too low for sensitive analysis. Instead, these are better detectable indirectly through a mechanism of fluorescence quenching in which each chromophore serves as a fluorescence quencher to a sensor molecule added separately such as coumarin [50]. This fluorescence quenching assay is validated with parathion, POX and fenitrothion, and it displays relatively low sensitivity in the range of 10−7–10−4 M [49].
2.2.1. Quantum Dot (QD) Nanosensors
QDs are notable for their bright fluorescence in the visible and near infrared (NIR) range [48][51]. Their fluorescence is applicable for OP detection as illustrated with QD sensors made of CdTe [52][48], CdS [53] and Mn-doped ZnS [54]. Their detection principle varies with specific design features introduced in each sensor, but it involves measuring a change in QD fluorescence intensity that occurs in response to OP adsorption or a chemical reaction on the QD surface [52]. The change occurs via either fluorescence resonance energy transfer (FRET) [48][53] or photoelectron transfer (PET) [54] between the donor (QD) and the OP-responsive acceptor attached on the surface. Zhang et al. reported dithizone-coordinated CdTe QD designed for FRET quenching-based chlorpyrifos detection via dithizone hydrolysis as shown in Figure 2B [48].
2.2.2. Upconversion Nanocrystal (UCN) Nanosensors
UCNs belong in an emerging class of photoactive nanomaterials that include NaYF4 doped with lanthanide ions (Yb, Er, Tm) in their lattice structure [55][56]. Unlike QDs, UCNs are excited by irradiation at longer NIR wavelengths (980 or 808 nm) with ability to emit upconversion luminescence at shorter visible wavelengths such as 475 nm [57]. Their luminescence intensity is sensitive to surface functionalization, and it can be quenched via its luminescence resonance energy transfer (LRET) to an acceptor molecule localized at a close proximity. In a recent study, Wang et al. [58] describes such luminescence quenching using UCN functionalized with an OP-reactive oxime probe on the surface (Figure 3A). This quenched luminescence is applicable for OP detection because it is restored when the oxime probe reacts with OP which leads to LRET deactivation. This UCN-based nanosensor has shown a detection sensitivity for diethyl chlorophosphate or dimethoate at μM [58].
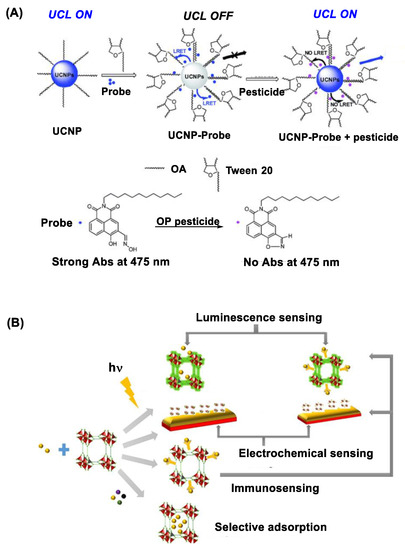
Figure 3. (A) Upconversion nanocrystal (UCN) immobilized with an oxime probe on the surface applied for OP detection through the mechanism of luminescence resonance energy transfer (LRET) between UCN and the oxime probe involved in organophosphate detection. Reproduced with permission from ref [58], Copyright 2016, The Royal Society of Chemistry. (B) A schematic diagram for metal-organic framework (MOF)-based approaches developed for pesticide sensing. Reproduced with permission from ref [59], Copyright 2018, American Chemical Society.
2.2.3. Metal-Organic Framework Nanosensors
OP can be detected by metal-organic frameworks (MOFs) that are active in UV photoluminescence. These include MOFs made of luminescent transition metal or lanthanide ions coordinated to organic ligands (imidazole) [60][61]. Their photoluminescence is highly responsive to microenvironmental changes in their lattice structure such as binding by guest molecules. Thus it is diminished to a significant extent upon OP binding or encapsulation (Figure 3B) [62]. This MOF-based luminescence assay enables to detect a broad spectrum of OP pesticides including chlorpyrifos, parathion and azinphos-methyl as described in a study by Singha et al. [63]. MOF sensors can be tunable in their design for improved guest specificity as reported with hafnium (Hf) ion-doped MOF [64]. In this study, Lian et al. describes its specific response to methanephosphonate, a hydrolytic byproduct from nerve agents, with high sensitivity. Other design factors in MOF sensors include those related to addressing potential drawbacks such as suboptimal aqueous stability, relatively slow onset of response and signal interference by other chemicals [59].
2.2.4. Plasmonic Nanomaterials
Noble metal nanomaterials that include nano gold (Au) or nano silver (Ag) display light absorbance via surface plasmon resonance (SPR) in the range of 350–500 nm (nano Ag) and 450–600 nm (nano Au)[24][65]. Their SPR absorbance is applicable for OP detection because it makes a blue shift upon chemisorption by sulfur analytes such as thiol-releasing OPs or thion (P=S)-based OPs (Table 1) [66]. Their detection sensitivity varies with metal compositions and shapes as evident with hexagon-shaped nano Ag which detects chlorpyrifos more effectively than other shapes [67].
Development of plasmonic nanosensors based on nano Au and nano Ag has certain limitations because they are not directly applicable for certain OPs that lack a sulfur moiety. Such lack of broader sensitivity is however addressed by surface functionalization with an OP-specific sensing element such as AChE [68], rhodamine B [69] or adenosine triphosphate [69]. Each of these sensors, which works in a different manner, has shown a broader sensitivity extended to oxon-based ethoprophos and dichlorvos [69][68] (Table 1).
In topic summary, several types of nanosensors are developed for OP analysis with improvement in detection time, sensitivity and specificity. Their capabilities are attributable to nanoscale structural and functional properties enabled by various types of nanomaterials that include MNPs [35][37][38], nano Au [39][69], nano Ag [40], MSN [41], graphene [42][44][45][46][47], QDs[52][48][54], luminescent UCN [58] and MOF [64]. These nanosensors are applicable for instrumental OP analysis by electrochemistry, SPR absorbance, fluorescence and luminescence.
This entry is adapted from the peer-reviewed paper 10.3390/nano11010224
References
- Guillaume Mercey; Tristan Verdelet; Julien Renou; Maria Kliachyna; Rachid Baati; Florian Nachon; Ludovic Jean; Pierre-Yves Renard; Reactivators of Acetylcholinesterase Inhibited by Organophosphorus Nerve Agents. Accounts of Chemical Research 2012, 45, 756-766, 10.1021/ar2002864.
- Eli Heldman; Yacov Ashani; Lily Raveh; Eliezer S. Rachaman; Sugar conjugates of pyridinium aldoximes as antidotes against organophosphate poisoning. Carbohydrate Research 1986, 151, 337-347, 10.1016/s0008-6215(00)90353-7.
- Sheng Xiong; Yaocheng Deng; Yaoyu Zhou; Daoxin Gong; Yuzhe Xu; Lihua Yang; Henghui Chen; Ling Chen; Tianwei Song; Ao Luo; et al. Current progress in biosensors for organophosphorus pesticides based on enzyme functionalized nanostructures: a review. Analytical Methods 2018, 10, 5468-5479, 10.1039/c8ay01851k.
- Donald W. Sparling; Ecotoxicology Essentials. Ecotoxicology Essentials 2015, Chapter 5, 109-152, 10.1016/c2014-0-00948-3.
- John E. Casida; Gary B. Quistad; Organophosphate Toxicology: Safety Aspects of Nonacetylcholinesterase Secondary Targets. Chemical Research in Toxicology 2004, 17, 983-998, 10.1021/tx0499259.
- Jaime D’Agostino; Haoming Zhang; Cesar Kenaan; Paul F. Hollenberg; Mechanism-Based Inactivation of Human Cytochrome P450 2B6 by Chlorpyrifos. Chemical Research in Toxicology 2015, 28, 1484-1495, 10.1021/acs.chemrestox.5b00156.
- Vivian S. Lin; Regan F. Volk; Adrian J. DeLeon; Lindsey N. Anderson; Samuel Owen Purvine; Anil K. Shukla; Hans Christopher Bernstein; Jordan N. Smith; Aaron T. Wright; Structure Dependent Determination of Organophosphate Targets in Mammalian Tissues Using Activity-Based Protein Profiling. Chemical Research in Toxicology 2019, 33, 414-425, 10.1021/acs.chemrestox.9b00344.
- Lawrence M. Schopfer; ‡ Troy Voelker; † Cynthia F. Bartels; ‡ And Charles M. Thompson; Oksana Lockridge†; Reaction Kinetics of Biotinylated Organophosphorus Toxicant, FP-biotin, with Human Acetylcholinesterase and Human Butyrylcholinesterase. Chemical Research in Toxicology 2005, 18, 747-754, 10.1021/tx049672j.
- Matthew C. Franklin; Michael J. Rudolph; Christopher Ginter; Michael S. Cassidy; Jonah Cheung; Structures of paraoxon-inhibited human acetylcholinesterase reveal perturbations of the acyl loop and the dimer interface. Proteins: Structure, Function, and Bioinformatics 2016, 84, 1246-1256, 10.1002/prot.25073.
- Pamela T. Wong; Somnath Bhattacharjee; Jayme Cannon; Shengzhuang Tang; Kelly Yang; Sierra Bowden; Victoria Varnau; Jessica J. O'konek; Seok Ki Choi; Reactivity and mechanism of α-nucleophile scaffolds as catalytic organophosphate scavengers. Organic & Biomolecular Chemistry 2019, 17, 3951-3963, 10.1039/c9ob00503j.
- Ki-Hyun Kim; Ehsanul Kabir; Shamin Ara Jahan; Exposure to pesticides and the associated human health effects. Science of The Total Environment 2016, 575, 525-535, 10.1016/j.scitotenv.2016.09.009.
- Omid C. Farokhzad; Robert Langer; Impact of Nanotechnology on Drug Delivery. ACS Nano 2008, 3, 16-20, 10.1021/nn900002m.
- Pamela T. Wong; Seok Ki Choi; Mechanisms of Drug Release in Nanotherapeutic Delivery Systems. Chemical Reviews 2015, 115, 3388-3432, 10.1021/cr5004634.
- Jolanta F. Kukowska-Latallo; Kimberly A. Candido; Zhengyi Cao; Shraddha S. Nigavekar; Istvan J. Majoros; Thommey P. Thomas; Lajos P. Balogh; Mohamed K. Khan; James R. Baker; Nanoparticle Targeting of Anticancer Drug Improves Therapeutic Response in Animal Model of Human Epithelial Cancer. Cancer Research 2005, 65, 5317-5324, 10.1158/0008-5472.can-04-3921.
- Pamela T. Wong; Dexin Chen; Shengzhuang Tang; Sean Yanik; Michael Payne; Jhindan Mukherjee; Alexa Coulter; Kenny Tang; Ke Tao; Kang Sun; et al. Modular Integration of Upconverting Nanocrystal-Dendrimer Composites for Folate Receptor-Specific NIR Imaging and Light-Triggered Drug Release. Small 2015, 11, 6078-6090, 10.1002/smll.201501575.
- Sarit S. Agasti; Subinoy Rana; Myoung-Hwan Park; Chae Kyu Kim; Chang-Cheng You; Vincent M. Rotello; Nanoparticles for detection and diagnosis. Advanced Drug Delivery Reviews 2010, 62, 316-328, 10.1016/j.addr.2009.11.004.
- Vasudevanpillai Biju; Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chemical Society Reviews 2013, 43, 744-764, 10.1039/c3cs60273g.
- Seok Ki Choi; Photoactivation Strategies for Therapeutic Release in Nanodelivery Systems. Advanced Therapeutics 2020, 3, 2000117, 10.1002/adtp.202000117.
- Seok Ki Choi; Activation Strategies in Image-Guided Nanotherapeutic Delivery. Journal of Nanotheranostics 2020, 1, 78-104, 10.3390/jnt1010007.
- † Matthew E. Stewart; † Christopher R. Anderton; † Lucas B. Thompson; ‡ Joana Maria; § Stephen K. Gray; † John A. Rogers; † Ralph G. Nuzzo; Nanostructured Plasmonic Sensors. Chemical Reviews 2008, 108, 494-521, 10.1021/cr068126n.
- Qingxin Mu; Guibin Jiang; Lingxin Chen; Hongyu Zhou; Denis Fourches; Alexander Tropsha; Bing Yan; Chemical Basis of Interactions Between Engineered Nanoparticles and Biological Systems. Chemical Reviews 2014, 114, 7740-7781, 10.1021/cr400295a.
- Yi Zhang; Yuhong Bai; Jianbo Jia; Ningning Gao; Yang Li; Ruinan Zhang; Guibin Jiang; Bing Yan; Perturbation of physiological systems by nanoparticles. Chemical Society Reviews 2013, 43, 3762-3809, 10.1039/c3cs60338e.
- Michael Dahl; Yiding Liu; Yadong Yin; Composite Titanium Dioxide Nanomaterials. Chemical Reviews 2014, 114, 9853-9889, 10.1021/cr400634p.
- Marie-Christine Daniel; Didier Astruc; Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chemical Reviews 2003, 104, 293-346, 10.1021/cr030698+.
- Dominik Eder; Carbon Nanotube−Inorganic Hybrids. Chemical Reviews 2010, 110, 1348-1385, 10.1021/cr800433k.
- Guanying Chen; Hailong Qiu; Paras N. Prasad; Xiaoyuan Chen; Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chemical Reviews 2014, 114, 5161-5214, 10.1021/cr400425h.
- Markus Haase; Helmut Schäfer; Upconverting Nanoparticles. Angewandte Chemie International Edition 2011, 50, 5808-5829, 10.1002/anie.201005159.
- Younan Xia; Weiyang Li; Claire M. Cobley; Jingyi Chen; Xiaohu Xia; Qiang Zhang; Miaoxin Yang; Eun Chul Cho; Paige K. Brown; Gold Nanocages: From Synthesis to Theranostic Applications. Accounts of Chemical Research 2011, 44, 914-924, 10.1021/ar200061q.
- Pamela T. Wong; Shengzhuang Tang; Jayme Cannon; Kelly Yang; Racquel Harrison; Matthew Ruge; Jessica J. O’Konek; Seok Ki Choi; Shielded α-Nucleophile Nanoreactor for Topical Decontamination of Reactive Organophosphate. ACS Applied Materials & Interfaces 2020, 12, 33500-33515, 10.1021/acsami.0c08946.
- Zhiqing Pang; Che-Ming J. Hu; Ronnie H. Fang; Brian T. Luk; Weiwei Gao; Fuqiang Wang; Erdembileg Chuluun; Pavimol Angsantikul; Soracha Thamphiwatana; Wenzhong Lu; et al. Detoxification of Organophosphate Poisoning Using Nanoparticle Bioscavengers. ACS Nano 2015, 9, 6450-6458, 10.1021/acsnano.5b02132.
- Huangxian Ju; Vivek Babu Kandimalla; Biosensors for pesticides. Electrochemical Biosensors 2007, Chapter 2, 31-56, 10.1016/b978-012373738-0.50004-0.
- Gustavo A. Alonso; Roberto Muñoz; Jean-Louis Marty; Automatic Electronic Tongue for On-Line Detection and Quantification of Organophosphorus and Carbamate Pesticides Using Enzymatic Screen Printed Biosensors. Analytical Letters 2013, 46, 1743-1757, 10.1080/00032719.2012.745087.
- Montserrat Cortina; Manuel Del Valle; Jean-Louis Marty; Electronic Tongue Using an Enzyme Inhibition Biosensor Array for the Resolution of Pesticide Mixtures. Electroanalysis 2007, 20, 54-60, 10.1002/elan.200704087.
- Xiaojuan Liu; Mengmeng Song; Ting Hou; Feng Li; Label-Free Homogeneous Electroanalytical Platform for Pesticide Detection Based on Acetylcholinesterase-Mediated DNA Conformational Switch Integrated with Rolling Circle Amplification. ACS Sensors 2017, 2, 562-568, 10.1021/acssensors.7b00081.
- Núbia Fernanda Marinho Rodrigues; Sakae Yotsumoto Neto; Rita De Cássia Silva Luz; Flávio Santos Damos; Hideko Yamanaka; Ultrasensitive Determination of Malathion Using Acetylcholinesterase Immobilized on Chitosan-Functionalized Magnetic Iron Nanoparticles. Biosensors 2018, 8, 16, 10.3390/bios8010016.
- A.Y. El-Moghazy; E.A. Soliman; H.Z. Ibrahim; T. Noguer; J.-L. Marty; G. Istamboulie; Ultra-sensitive biosensor based on genetically engineered acetylcholinesterase immobilized in poly (vinyl alcohol)/Fe–Ni alloy nanocomposite for phosmet detection in olive oil. Food Chemistry 2016, 203, 73-78, 10.1016/j.foodchem.2016.02.014.
- Georges Istamboulie; Silvana Andreescu; Jean-Louis Marty; Thierry Noguer; Highly sensitive detection of organophosphorus insecticides using magnetic microbeads and genetically engineered acetylcholinesterase. Biosensors and Bioelectronics 2007, 23, 506-512, 10.1016/j.bios.2007.06.022.
- Rocio B. Dominguez; Gustavo A. Alonso; Roberto Muñoz; Akhtar Hayat; Jean-Louis Marty; Design of a novel magnetic particles based electrochemical biosensor for organophosphate insecticide detection in flow injection analysis. Sensors and Actuators B: Chemical 2015, 208, 491-496, 10.1016/j.snb.2014.11.069.
- Nidhi Chauhan; Jagriti Narang; C.S. Pundir; Immobilization of rat brain acetylcholinesterase on porous gold-nanoparticle–CaCO3 hybrid material modified Au electrode for detection of organophosphorous insecticides. International Journal of Biological Macromolecules 2011, 49, 923-929, 10.1016/j.ijbiomac.2011.08.006.
- QiQi Zheng; Yonghua Yu; Kai Fan; Feng Ji; Jian Wu; Yibin Ying; A nano-silver enzyme electrode for organophosphorus pesticide detection. Analytical and Bioanalytical Chemistry 2016, 408, 5819-5827, 10.1007/s00216-016-9694-6.
- Jeyanthi Palanivelu; Ramalingam Chidambaram; Acetylcholinesterase with mesoporous silica: Covalent immobilization, physiochemical characterization, and its application in food for pesticide detection. Journal of Cellular Biochemistry 2019, 120, 10777-10786, 10.1002/jcb.28369.
- Jyotsana Mehta; Priya Vinayak; Satish K. Tuteja; Varun A. Chhabra; Neha Bhardwaj; A.K. Paul; Ki-Hyun Kim; Akash Deep; Graphene modified screen printed immunosensor for highly sensitive detection of parathion. Biosensors and Bioelectronics 2016, 83, 339-346, 10.1016/j.bios.2016.04.058.
- Yu Chen; Chaoliang Tan; Hua Zhang; Lianzhou Wang; Two-dimensional graphene analogues for biomedical applications. Chemical Society Reviews 2014, 44, 2681-2701, 10.1039/c4cs00300d.
- Zhi Li; Heji Zhang; Xueping Ge; Ying Liang; Xingcai An; Cunzhong Yang; Bin Fang; Haifen Xie; Jianjun Wei; A nanocomposite of copper(ii) functionalized graphene and application for sensing sulfurated organophosphorus pesticides. New Journal of Chemistry 2012, 37, 3956, 10.1039/c3nj00528c.
- Murilo H.M. Facure; Luiza A. Mercante; Luiz H.C. Mattoso; Daniel S. Correa; Detection of trace levels of organophosphate pesticides using an electronic tongue based on graphene hybrid nanocomposites. Talanta 2017, 167, 59-66, 10.1016/j.talanta.2017.02.005.
- Jingming Gong; Xingju Miao; Ting Zhou; Lizhi Zhang; An enzymeless organophosphate pesticide sensor using Au nanoparticle-decorated graphene hybrid nanosheet as solid-phase extraction. Talanta 2011, 85, 1344-1349, 10.1016/j.talanta.2011.06.016.
- Wu Huixiang; Huo Danqun; Zhao Yanan; Ma Na; Hou Jingzhou; Liu Miao; Shen Caihong; Hou Changjun; A non-enzymatic electro-chemical sensor for organophosphorus nerve agents mimics and pesticides detection. Sensors and Actuators B: Chemical 2017, 252, 1118-1124, 10.1016/j.snb.2017.07.004.
- Kui Zhang; Qingsong Mei; Guijian Guan; Bianhua Liu; Suhua Wang; Zhongping Zhang; Ligand Replacement-Induced Fluorescence Switch of Quantum Dots for Ultrasensitive Detection of Organophosphorothioate Pesticides. Analytical Chemistry 2010, 82, 9579-9586, 10.1021/ac102531z.
- Sheetal Paliwal; Melinda Wales; Theresa Good; Janet Grimsley; James Wild; Aleksandr Simonian; Fluorescence-based sensing of p-nitrophenol and p-nitrophenyl substituent organophosphates. Analytica Chimica Acta 2007, 596, 9-15, 10.1016/j.aca.2007.05.034.
- Sherine O. Obare; Chandrima De; Wen Guo; Tajay L. Haywood; Tova A. Samuels; Clara P. Adams; Noah O. Masika; Desmond H. Murray; Ginger A. Anderson; Keith Campbell; et al. Fluorescent Chemosensors for Toxic Organophosphorus Pesticides: A Review. Sensors 2010, 10, 7018-7043, 10.3390/s100707018.
- Juan Zhou; Yong Yang; Chun-Yang Zhang; Toward Biocompatible Semiconductor Quantum Dots: From Biosynthesis and Bioconjugation to Biomedical Application. Chemical Reviews 2015, 115, 11669-11717, 10.1021/acs.chemrev.5b00049.
- Tao Yu; Tian-Yi Ying; Yun-Yang Song; Yan-Jun Li; Fang-Hui Wu; Xiao-Qiang Dong; Jiang-Shan Shen; A highly sensitive sensing system based on photoluminescent quantum dots for highly toxic organophosphorus compounds. RSC Advances 2013, 4, 8321-8327, 10.1039/c3ra47519k.
- Pijush Ch. Dey; Ratan Das; Ligand free surface of CdS nanoparticles enhances the energy transfer efficiency on interacting with Eosin Y dye – Helping in the sensing of very low level of chlorpyrifos in water. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2019, 207, 156-163, 10.1016/j.saa.2018.09.014.
- Kui Zhang; Tao Yu; Fei Liu; Mingtai Sun; Huan Yu; Bianhua Liu; Zhongping Zhang; Hui Jiang; Suhua Wang; Selective Fluorescence Turn-On and Ratiometric Detection of Organophosphate Using Dual-Emitting Mn-Doped ZnS Nanocrystal Probe. Analytical Chemistry 2014, 86, 11727-11733, 10.1021/ac503134r.
- Niagara Muhammad Idris; Muthu Kumara Gnanasammandhan Jayakumar; Akshaya Bansal; Yong Zhang; Upconversion nanoparticles as versatile light nanotransducers for photoactivation applications. Chemical Society Reviews 2014, 44, 1449-1478, 10.1039/c4cs00158c.
- Stephan Heer; Karsten Kompe; H.-U. Güdel; Michael A Haase; Highly Efficient Multicolour Upconversion Emission in Transparent Colloids of Lanthanide-Doped NaYF4Nanocrystals. Advanced Materials 2004, 16, 2102-2105, 10.1002/adma.200400772.
- Tao, K.; Sun, K.; Choi, S.K; Photonanotechnology for Therapeutics and Imaging. Photonanotechnology for Therapeutics and Imaging 2020, Chapter 12, 345-371, 10.1016/c2018-0-02259-8.
- Shuailiang Wang; Xiaobo Wang; Xingxiang Chen; Xiaozheng Cao; Jing Cao; Xiaofeng Xiong; Wenbin Zeng; A novel upconversion luminescence turn-on nanosensor for ratiometric detection of organophosphorus pesticides. RSC Advances 2016, 6, 46317-46324, 10.1039/c6ra05978c.
- Kumar Vikrant; Daniel C. W. Tsang; Nadeem Raza; Balendu Shekher Giri; Deepak Kukkar; Ki-Hyun Kim; Potential Utility of Metal–Organic Framework-Based Platform for Sensing Pesticides. ACS Applied Materials & Interfaces 2018, 10, 8797-8817, 10.1021/acsami.8b00664.
- Sarita Dhaka; Rahul Kumar; Akash Deep; Mayur B. Kurade; Sang-Woo Ji; Byong-Hun Jeon; Metal–organic frameworks (MOFs) for the removal of emerging contaminants from aquatic environments. Coordination Chemistry Reviews 2019, 380, 330-352, 10.1016/j.ccr.2018.10.003.
- Bing Yan; Lanthanide-Functionalized Metal–Organic Framework Hybrid Systems To Create Multiple Luminescent Centers for Chemical Sensing. Accounts of Chemical Research 2017, 50, 2789-2798, 10.1021/acs.accounts.7b00387.
- Kumar Vikrant; Daniel C. W. Tsang; Nadeem Raza; Balendu Shekher Giri; Deepak Kukkar; Ki-Hyun Kim; Potential Utility of Metal–Organic Framework-Based Platform for Sensing Pesticides. ACS Applied Materials & Interfaces 2018, 10, 8797-8817, 10.1021/acsami.8b00664.
- Debal Kanti Singha; Prakash Majee; Sudip Kumar Mondal; Partha Mahata; Highly Selective Aqueous Phase Detection of Azinphos-Methyl Pesticide in ppb Level Using a Cage-Connected 3D MOF. ChemistrySelect 2017, 2, 5760-5768, 10.1002/slct.201700963.
- Xiao Lian; Bing Yan; Trace Detection of Organophosphorus Chemical Warfare Agents in Wastewater and Plants by Luminescent UIO-67(Hf) and Evaluating the Bioaccumulation of Organophosphorus Chemical Warfare Agents. ACS Applied Materials & Interfaces 2018, 10, 14869-14876, 10.1021/acsami.8b00289.
- Huanjun Chen; Lei Shao; Qian Li; Jianfang Wang; Gold nanorods and their plasmonic properties. Chemical Society Reviews 2012, 42, 2679-2724, 10.1039/c2cs35367a.
- Niluka M. Dissanayake; Jaliya S. Arachchilage; Tova A. Samuels; Sherine O. Obare; Highly sensitive plasmonic metal nanoparticle-based sensors for the detection of organophosphorus pesticides. Talanta 2019, 200, 218-227, 10.1016/j.talanta.2019.03.042.
- Sumit Sarkar; Ratan Das; Presence of chlorpyrifos shows blue shift of the absorption peak of silver nanohexagons solution – An indication of etching of nanocrystals and sensing of chlorpyrifos. Sensors and Actuators B: Chemical 2018, 266, 149-159, 10.1016/j.snb.2018.03.123.
- Yong Liu; Bingjing Lv; Anran Liu; Geyu Liang; Lihong Yin; Yuepu Pu; Wei Wei; Shaohua Gou; Songqin Liu; Multicolor sensor for organophosphorus pesticides determination based on the bi-enzyme catalytic etching of gold nanorods. Sensors and Actuators B: Chemical 2018, 265, 675-681, 10.1016/j.snb.2018.03.023.
- Xiaoxia Li; Haixin Cui; Zhanghua Zeng; A Simple Colorimetric and Fluorescent Sensor to Detect Organophosphate Pesticides Based on Adenosine Triphosphate-Modified Gold Nanoparticles. Sensors 2018, 18, 4302, 10.3390/s18124302.
- Sarita Dhaka; Rahul Kumar; Akash Deep; Mayur B. Kurade; Sang-Woo Ji; Byong-Hun Jeon; Metal–organic frameworks (MOFs) for the removal of emerging contaminants from aquatic environments. Coordination Chemistry Reviews 2019, 380, 330-352, 10.1016/j.ccr.2018.10.003.
- Bing Yan; Lanthanide-Functionalized Metal–Organic Framework Hybrid Systems To Create Multiple Luminescent Centers for Chemical Sensing. Accounts of Chemical Research 2017, 50, 2789-2798, 10.1021/acs.accounts.7b00387.
- Debal Kanti Singha; Prakash Majee; Sudip Kumar Mondal; Partha Mahata; Highly Selective Aqueous Phase Detection of Azinphos-Methyl Pesticide in ppb Level Using a Cage-Connected 3D MOF. ChemistrySelect 2017, 2, 5760-5768, 10.1002/slct.201700963.
- Huanjun Chen; Lei Shao; Qian Li; Jianfang Wang; Gold nanorods and their plasmonic properties. Chemical Society Reviews 2012, 42, 2679-2724, 10.1039/c2cs35367a.
- Niluka M. Dissanayake; Jaliya S. Arachchilage; Tova A. Samuels; Sherine O. Obare; Highly sensitive plasmonic metal nanoparticle-based sensors for the detection of organophosphorus pesticides. Talanta 2019, 200, 218-227, 10.1016/j.talanta.2019.03.042.
- Sumit Sarkar; Ratan Das; Presence of chlorpyrifos shows blue shift of the absorption peak of silver nanohexagons solution – An indication of etching of nanocrystals and sensing of chlorpyrifos. Sensors and Actuators B: Chemical 2018, 266, 149-159, 10.1016/j.snb.2018.03.123.
- Yong Liu; Bingjing Lv; Anran Liu; Geyu Liang; Lihong Yin; Yuepu Pu; Wei Wei; Shaohua Gou; Songqin Liu; Multicolor sensor for organophosphorus pesticides determination based on the bi-enzyme catalytic etching of gold nanorods. Sensors and Actuators B: Chemical 2018, 265, 675-681, 10.1016/j.snb.2018.03.023.
