Biosurfactants can play a significant role in the prevention, control and treatment of diseases caused by a range of pathogenic agents through various therapeutic, pharmaceutical, environmental and hygiene approaches.
- biosurfactants
- antiviral
- antimicrobial
- COVID-19
1. Biosurfactants
Biosurfactants are amphiphilic compounds synthesized by bacteria, fungi, or plants, capable of lowering the surface tension of liquids. Their hydrophilic moieties can be made of acid, cationic peptide, anion, sugar (monosaccharide, disaccharide, or polysaccharide) and hydrophobic moieties made of hydrocarbon or fatty acid chains. They are environmentally friendly and have been shown to have many industrial applications sometimes performing better than synthetic surfactants [1][2][3][4][5]. Their physico-chemical properties include; high stability in a wide range of environmental conditions such as extreme pH, temperature and also salt concentration [6][7], high biodegradability with a high rate of mineralization by soil microcosms [8], low toxicity, surface tension reduction, foaming capacity and antimicrobial activity against pathogens [9][10][11][12].
Well known classifications of biosurfactants are based on their charges and molecular structures. Biosurfactants obtained from microbes are generally anionic or neutral, while a few are cationic [5]. Long-chain fatty acids generally characterize the hydrophobic moiety, while organic acid, alcohol, amino acid or carbohydrate functional groups characterize the hydrophilic moiety [5]. They are also broadly grouped based on their chemical structures as high molecular weight and low molecular weight molecules. Low molecular weight molecules are the glycolipids, phospholipids and lipopeptides, while the high molecular weight groups are the polymeric and particulate biosurfactants [13] (Figure 1). In addition to their environmental friendliness and wide industrial applications, biosurfactants are more sustainable because they can be produced from cheap feedstock and industrial and urban waste, and can be recycled [14][15]. However, there are still challenges such as the pathogenicity of the main biosurfactant producer strains Pseudomonas and Bacillus and the relatively higher cost of large-scale production that make biosurfactants less commercially competitive than petroleum-derived synthetic surfactants [16]. Nevertheless, there is a growing scientific research interest in the improvement of the commercial competitiveness of biosurfactants. Bacterial strains are being engineered, new feedstocks are tested, and fermentation designs investigated to develop innovative ways to improve production efficiency. Amidst these challenges, it is expected that biosurfactants will occupy a significant market share, which is projected to be about USD 5.52 billion by 2022, growing at a CAGR (compound annual growth rate) of 5.6% [17].
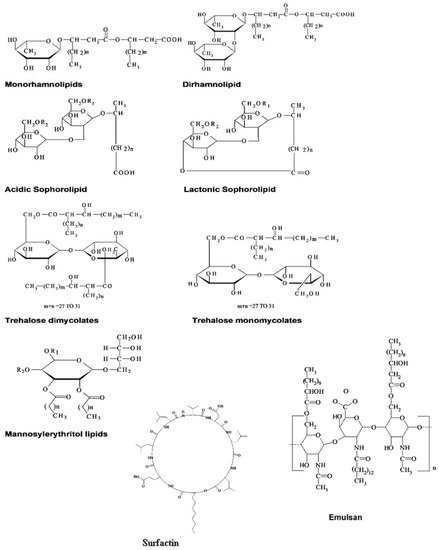
Figure 1. Representative biosurfactants produced by microorganisms utilizing water-soluble and /or water-insoluble substrates [1].
2. Pathogens and Other Agents with Outbreak or Pandemic Potential
RNA viruses are the class with the highest risks of an outbreak especially those that are airborne such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), because the mere act of breathing spreads the virus. Other organisms could potentially evolve or be engineered through drug resistance or human manipulation. With these factors in mind, the development of pipelines for both broad-spectrum and specific therapeutics could be important to add resilience against outbreak-causing pathogens. The first line of treatment for these emerging diseases can be broad-spectrum antimicrobials, while specific treatment options could benefit from modifications that diversify the mechanism of action of such therapeutic molecules. This can also be integrated with other environmental approaches that disrupt transmission from the common source of spread in the population [18]. According to a report by the Intergovernmental Platform on Biodiversity and Ecosystem Services, there are over 1.7 million viruses unknown to man, with 540–850 thousand that can potentially infect humans. The cost of risk mitigation for these pandemics could be 100 times less than the cost of pandemic management. If the status quo on the fight of infectious diseases is not changed, we may see more outbreaks in the future with more damaging effects [19]. Some currently known diseases with high pandemic or outbreak importance include; Crimean–Congo hemorrhagic fever, chikungunya, cholera, Ebola virus disease, influenza (seasonal, zoonotic, pandemic), Hendra virus infection, Lassa fever, meningitis, Marburg virus disease, MERS-CoV, Nipah virus infection, monkeypox, novel coronavirus (2019-nCoV), SARS, smallpox, Tularaemia, plague, Rift Valley fever, Yellow fever and Zika virus disease [20]. Important bacterial-resistant strains include Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae and Mycobacterium tuberculosis, among others [18].
3. Approaches That Can Use Biosurfactants in Outbreak Prevention and Management
There are various areas where biosurfactants can be used as alternative sustainable solutions or as innovative components applicable in outbreak prevention and management. These include uses in therapeutics (vaccine development, immune system enhancers and drug delivery and developments), environmental applications as agricultural and pest biopesticides, and various industrial sectors as surface cleaning agents, disinfectants, detergents, and the packaging industries. All these applications are summarized below in Figure 2, and further discussed in the following subsections.
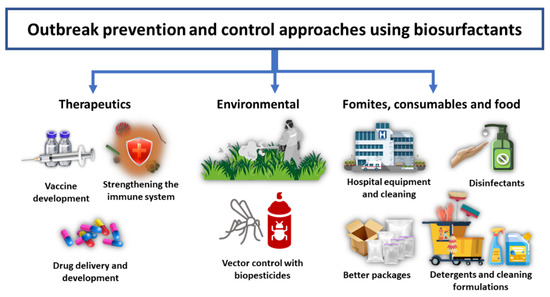
Figure 2. A summary of potential approaches for applications of biosurfactants in future outbreak prevention and control.
3.1 Therapeutics
Treatment availability is the most important element for the elimination of an outbreak, because the pathogen can be eliminated, thereby interrupting the transmission cycle and impeding the spread. However, the development of an effective drug against a disease takes years due to the required clinical trials and approval processes. Using already-approved low toxicity molecules or repurposing existing drugs to target a new pathogen can contribute to the development of effective therapy in a shorter time. Biosurfactants are one of the most promising biomolecules in the pharmaceutical industry because of their structural versatility, stability, micelle forming ability, biological compatibility and low toxicity that are useful in the design of therapeutics. They can be safely used for oral, nasal, or dermal applications [21]. Another important characteristic of biosurfactants is their ability to interact with surfaces such as the membranes of organisms or with their surrounding environment, which can be useful in intracellular targeting.
Lipopeptides, glycolipids, and Mannosylerythritol lipids produced from Candida species are the most common microbial surfactants that have been investigated for pharmaceutical applications. They are relevant in pharmaceuticals for the delivery of drugs and genes to target cells, the design of molecules to interact with components of the immune system, and as antimicrobial agents. Biocompatibility, low toxicity, and environmental friendliness are advantages that make them better options over synthetic surfactants [16][22][23][24]. Biosurfactants have various innovative applications in the development of effective therapeutics which can come in handy at times of urgent need, such as during outbreaks or pandemics.
3.1.1 Delivery Systems
Delivery systems are structures to improve the effectiveness of drugs, but challenges such as poor delivery due to dilution by biological fluids and drug precipitation are amongst the main reasons for various failures in drug delivery system designs. Challenges faced when surfactants are used as delivery systems include the stability, packaging and formulations providing proper solubility in lipids, low drug loading capacity and risks of gastrointestinal irritations due to high amounts used [25].
Biosurfactants can form self-aggregating structures called micelles (Figure 3), which can act as good emulsifiers and have antimicrobial properties useful in the design of drug delivery systems. Biosurfactant-based microemulsion drug delivery systems can be used to make existing therapeutics more effective by either increasing the loading capacity, bioavailability, or provide more control in the release. They can encapsulate and solubilize hydrophobic and hydrophilic drugs in emulsions [26]. Microemulsion drug delivery systems are bio-compatible, thermodynamically stable, and usually highly surface active. Glycolipids and lipopeptides have been commonly used for this purpose and are a potential replacement for contemporary synthetic options [27]. These versatile microemulsion systems are emerging as novel drug delivery systems that can enable the modification of drug formulations for topical, oral, nasal, ocular, intravenous, or other routes of drug administration[25][26].
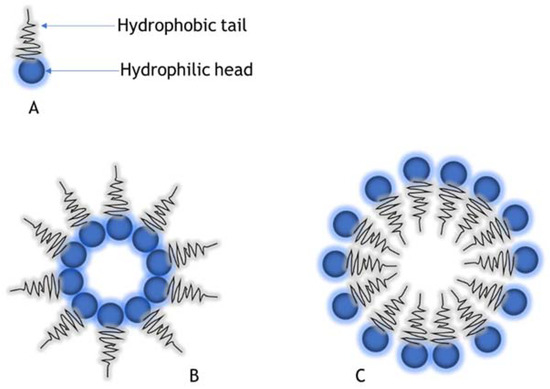
Figure 3. (A) Biosurfactant molecule, (B) Reverse micelle structure, (C) Micelle structure.
Glycolipid sugar chains can also be used to target specific cells because they can be recognizable by carbohydrate-binding proteins on cell surfaces[28]. Through precise modifications, this can be applied in targeting specific cells with intracellular pathogens such as viruses, thereby reducing spread to other less-affected cells. Glycolipid micelles can form structures such as liposomes, gels, niosomes, cubosomes and hexosomes[28] which can serve as a vehicle for the delivery of a drug to a target site while maintaining its integrity and that of the molecule carried in the body fluid environment. In the case of COVID-19, an aerosol formulation containing biosurfactant drug delivery system has been suggested to be a likely mode to deliver drugs, because the virulence is mainly observed in the lungs. In addition to delivery, another advantage is their antiviral activity which can inhibit viruses at the locus of infection[29]. In nanobiotechnology, applications of rhamnolipids as microemulsion stabilizers of different nanoparticles in drug delivery systems have also been reported. These include nickel oxide nanoparticles[30][31] and silver nanoparticles[32][33][34][35]. It is clear that biosurfactant-based drug delivery systems have the potential for better efficiency in drug delivery systems; research has increased around the exploration of the effects on virulent microorganism species that have outbreak potential.
3.1.2. Vaccines and Immunity
Coronavirus diseases such as severe acute respiratory syndrome (2002–2003) and Middle East respiratory syndrome (2011) were completely new agents of zoonotic origin. With COVID-19 infections, a normally functioning immune system clears the virus completely without the person developing symptoms. The innate and adaptive immunity play a primary role in this process because the immune system is still unfamiliar with the pathogen and is unable to work properly in some cases. Priming or inducing immune cells (T cells, B cells, macrophages, neutrophils, etc.) is crucial for an effective adaptive response. When T cells are primed, they clear viral infected cells through a cellular immune response as well as mediating the differentiation of B cells into plasma cells and memory cells which, respectively, produce viral-specific antibodies and store a memory of the response in case the infection returns[36]. Therefore, for new infections, adaptive immunity plays an important role through T cell activation. One way to safely activate T cells is by using vaccines. Peptide antigen vaccines are effective because they can be obtained at high purity compared to whole organisms or protein vaccines. However, they are limited by low immunogenicity.
Bacterial lipopeptides can be potent nontoxic, nonpyrogenic immunological adjuvants when coupled with antigens. They activate the immune system by signaling through toll-like receptor 2 (TLR2). Activity can vary considerably with different structures of lipopeptides providing great flexibility in the design of vaccine systems[37]. Deres and colleagues used lipopeptides as adjuvants with viral peptides to prime viral-specific cytotoxic T cells which are a component of the cellular immune response against viral infections. For activation to occur, T cells must recognize peptides from the virus coupled with the MHC (major histocompatibility complex) class I molecules displayed by cells infected by the virus. Tripalmitoyl-S-glycerylcysteinyl-seryl-serine lipopeptide has been covalently attached to a synthetic viral peptide which produced the same cytotoxic T cells mediated immune response observed with a live and infectious virus[38]. These formulations can prove to be effective in cases where there is no initial immunity to a pathogen, or as a resort to boost immunity in combination with other therapy.
The development of effective formulations with adjuvants that can safely boost immunity and enhance activity is a major challenge in vaccine development[39]. Some previous studies have investigated the role of surfactants in defense using bioactive peptides for the inactivation of enveloped viruses. The viral cycle of the influenza virus, for example, was reportedly inhibited by Cyclosporin A biopeptide produced by a fungus known as Tolypocladium inflatum through impeding viral assembly, or the budding (exiting step), after protein synthesis[40]. It was hypothesized that targeting these latter stages in the viral life cycle can overcome the problem of resistance for antiviral drugs, and hence limit disease spread.
3.1.3. Inflammation
COVID-19 infections are characterized by pulmonary and systemic inflammatory responses which cause cytokine storms. This results in hypersensitivity and the death of other healthy cells, leading to the manifestation of severe acute respiratory distress syndrome and disseminated intravascular coagulation, which are the main causes of mortality during infection. These storms may be because of an increase in the levels of inflammatory molecules such as IL-2, TNF-α and IL-6. Meanwhile, a low inflammatory response may promote a moderate T cell response[41]. The anti-inflammatory and antiviral role of biosurfactants has been demonstrated through cytokines (TNF-α, IL-6, IL-8, IL-12, Il-18 and IL-1β) and toll-like receptors-2 (TLR-2). After an inflammatory response, these factors can cause the secretion of proteins with cationic charge and other reactive oxygen species, including lysozyme, which can be used for therapeutic purposes. The reactive oxygen species produced have anti-inflammatory and antiviral properties which can be used as therapeutic agents against viral diseases. These can be used in the management of the cytokine storm, which is a cause of damages in the lungs as found in many COVID-19 patients. Although this hypothesis has not yet been tested or confirmed, it has been advanced as a possible mechanism which could be applied for the development of therapeutics against viral infections including COVID-19[42].
3.1.4. Enzymes and Biocatalysts
There is a growing use of colloids in chemistry and biotechnology, particularly enzyme-containing reversed micellar systems. Biosurfactants are molecules with both hydrophobic and hydrophilic moieties, which, when introduced in nonpolar organic solvents, can self-aggregate when their concentration is greater than the critical micelle concentration. This aggregated structure contains an inner polar core made from polar structures and an outer nonpolar part consisting of hydrocarbon tails. The inner core provides a structure in which nano-sized particles can be contained and thermodynamically stabilized. Additionally, these structures are transparent enough to be seen under microscopes. It was also discovered that this inner environment mimics the internal environment of cells, which could be an explanation for the increase in enzyme activity. DNA cleavage enzymes have been tested both in vivo and in vitro, showing disruption in essential genes required for replication in Hepatitis B, HIV, HPV, HSV, HTLV viral infections[43]. The increasing use of CRISPR Cas technology in therapy shows that gene therapy will increasingly be used in many diseases. Enzyme delivery systems can be useful options in the treatment or delivery of enzyme components to the required target site. This approach could provide an opportunity to diversify the portfolio and pipelines for the treatment options considered for COVID-19 or other serious infections. There are numerous other applications in medicine, especially for protein extraction and bioactive molecule separation and purification, as well as enzyme drug delivery[44].
3.1.5. Probiotics
Probiotics are living bacteria that have health benefits to the human gut microbiota, either by improving or restoring it. Besides improving the gut microbiota, they can contribute to improving the immune system. Probiotic lactic acid bacteria have been reported to produce various biosurfactants with important applications in biotherapeutics due to their important antimicrobial and anti-adhesive activity. They can significantly improve the defense of the gastrointestinal microbiota in outbreaks that cause gastrointestinal diseases. The main producers of probiotics include Lactobacillus and Bifidobacterium. Other lactic acid bacteria include Enterococcus, Leuconostoc, Pediococcus, Sporolactobacillus, Streptococcus, Lactococcus, and non-lactic acid bacteria include Propionibacterium, Bacillus cereus, Saccharomyces, Bifidobacterium lactis, and Escherichia coli strain nissle[45].
Probiotic bacteria producing biosurfactants have shown anti-microbial activity against Gram-negative and Gram-positive bacteria, including yeast and other possible human pathogens[45]. These include Lactobacillus paracasei[46], glycolipid from Lactococcus lactis with activity against multidrug-resistant (MDR) pathogens[47], Lactobacillus helveticus[48], lipopeptide from Bacillus cereus NK1[49], L. paracasei ssp paracasei A20[50], Lactobacillus casei MRTL3[51] and Lactobacillus acidophilus[52][53].
There is some evidence that probiotics may be able to decrease the risk and duration of viral respiratory infections. This may, therefore, be helpful for the management of COVID-19 infections. Although the actual mechanism of action is undefined, clear benefits were demonstrated in some initial trials. It was hypothesized that this may be through direct interaction with the virus or stimulation of the immune system. Research to investigate specific viral effects, especially on RNA viruses and other respiratory viruses, is recommended because this could provide a safe method to fight related infections in emergencies[54].
3.1.6. Drug Development
Chemical modifications of biomolecules, especially lipids, have numerous applications in chemistry and pharmacokinetics. The hydrogen atom deuterium is a stable hydrogen isotope, having both a proton and a neutron within its nucleus. Deuterium oxide (D2O) is well known in medicinal chemistry, and it is being used in the modification of various biomolecules for pharmaceutical purposes[55]. Deuterated biosurfactants are produced by bacteria strain AD7 of Pseudomonas aeruginosa with varying levels of D2O and carbon substrates. These molecules are safer and can be used to monitor drug metabolism within biological systems. These are used as substrates, therefore the level of deuteration of the biosurfactant can be manipulated. The flexibility of a biosurfactant used as an adjuvant can enable modifications on antibiotics and other drugs, which can result in the improved performance of an existing antibiotic, or even restrict the development of antimicrobial resistance with the possibility of using a small dose of the drug[56]. Besides pharmacokinetics, the antimicrobial properties of biosurfactants can be exploited in drug development. Rhodococcus fascians BD8 isolated from artic soil was found to produce a trehalose lipid biosurfactant with antimicrobial activity against the drug-resistant bacteria Vibrio harveyi and Proteus vulgaris. Partial inhibition at 11–34% was observed on other Gram-positive and Gram-negative bacteria, as well as 30% inhibition of Candida albicans at 0.5 mg/mL concentration[57].
In combination with existing drugs, synergistic effects between biosurfactants and antibiotics have also been reported. Methicillin-resistant Staphylococcus aureus was inhibited by using a joint sophorolipid and tetracycline treatment in vitro. Bacteria inhibition was observed at concentrations below the minimum inhibitory concentration. Understanding the underlying mechanisms of synergy between biosurfactants and antibiotics could prove useful in the development of effective treatments against drug-resistant pathogens[58]. Another way to develop effective treatments is the use of precision antimicrobials. Their applications in personalized medicine as a way of reducing the effects of drugs in the body are rapidly expanding. This novel approach to treating infectious diseases can use biosurfactants[59].
Bacaucin is a peptide biosurfactant isolated from Bacillus subtilis strain CAU21, which was reported to have broad-spectrum antimicrobial properties against Gram-positive bacteria but with haemolytic and cytotoxic effects. When the lipid portion is removed and the ring of the heptapeptide opened, bacaucin-1 is produced, which has more hydrophilic portions exposed, resulting in an overall decrease in the hydrophobicity of the molecule. Bacaucin-1 was selectively active against antibiotic-resistant S. aureus strains through cell membrane disruption, and demonstrated no bacterial resistance and cytotoxicity to mammalian cells in vitro and in vivo[60]. Structural modification of biosurfactants could lead to the development of highly precise drugs which can be used against pathogens sharing many similarities with human cells.
Biosurfactants from Bacillus subtilis have also shown in vitro antiviral properties against the enveloped virus species such as herpes, retroviruses, and other non-enveloped viruses. These groups share structural similarity with pathogens such as HIV, MERS, SARS, and Hepatitis. Bacillus subtilis surfactin inactivated the viruses at concentrations of 25 µm–80 µm. The mechanism of action was through the destruction of viral lipid membranes and capsid[61]. The formation of ion channels in the viral capsids and lipid envelopes leading to the loss of proteins involved in the process of membrane attachment, fusion and penetration has been reported[62]. Other results have described the effects of surface-active lipopeptide mixtures and surfactin analogues against Newcastle disease virus and Porcine epidemic diarrhea virus, emphasizing the idea of potential use as new antiviral drugs[62][63][64]. The wide antiviral properties show their possible applications in various pharmaceutical formulations against enveloped viruses such as SARS-CoV-2.
3.2. Applications in Diagnostics
3.2.1. Nanomaterials and Nanotechnology
Biosensors (typically simple, sensitive, robust, and cost-effective) combined with nanomaterials, also known as nanobiosensors, can serve as a bridge between advanced diagnostics/detection and routine testing. It is essential that the production processes for nanoparticles become cleaner, less toxic, and more environmentally safe so that the negative impact on the environment from waste can be minimized. Microorganisms are capable of synthesizing inorganic molecules that can be deposited either intracellularly or excreted extracellularly. Sophorolipid-capped cobalt nanoparticles can be used to generate biocompatible particle surfaces by the attachment of bioactive molecules such as glycosidases or lectins for diagnostic and medicinal applications. An important issue of biocompatibility properties is the accessibility of the sophorose group at the surface of the nanoparticles[65]. Silver nanoparticles synthesized with purified rhamnolipids from P. aeruginosa BS-161R strain demonstrated broad-spectrum antimicrobial activity against Gram-negative and Gram-positive bacteria, and against Candida albicans[34].
Nanotechnology is a relatively new field which is growing very rapidly, and nanoparticles are being used in sophisticated devices and in treatment. As the need for nanoparticles grows, it will be necessary to make production environmentally friendly and sustainable. This can be achieved using the biosurfactant-mediated production of nanoparticles. Diagnostics using nanotechnology are the basis for highly sensitive and specific diagnostic devices that require very small sample amounts. During outbreaks, the availability of highly accurate and specific diagnostics is important in the proper identification of the causative agent, and subsequently building a response.
3.2.2. Contrasting Agents
Microbubbles synthesized with biosurfactants can offer an option for the synthesis or the development of non-invasive, low-cost, and highly specific diagnostics. They can be applied in diagnoses using molecular imaging. For the diagnosis of specific diseases, the bubble surface can be chemically modified or conjugated to a disease-specific ligand. When retained in the targeted tissue and detected with ultrasound by using it as a contrast, it can be a means for specific and sensitive diagnosis in early disease detection and progression[66][67].
3.3. Environmental Approaches
3.3.1. Environmental Control and Management against Potential Outbreaks
Wastewater remains a significant transmission route of pathogens that have an outbreak or pandemic potential. This poses a significant challenge, especially for health authorities when it comes to testing and understanding the transmission dynamics of pathogens. In the case of the current COVID-19 pandemic, the role of wastewater treatment in the transmission of the virus remains unknown[68]. Detecting pathogen DNA or RNA in wastewater samples can provide an early warning system in the transmission of the pathogen in the community. Therefore, early warning systems in wastewater treatment facilities using optimized protocols for sampling, sample storage and recommended concentrations of genetic material can be an important tool for the early detection, response, and management of an outbreak[69]. Biosurfactants have been shown to have broad-spectrum antimicrobial activity, therefore their applications in the treatment of wastewater can serve as an early and safe method for managing sewage in sewage treatment facilities[70].
Wastewater pollution can be a cause of thalassogenic infectious diseases when poorly treated wastewater is disposed into the sea. The WHO estimates that, globally, 120 million cases of gastrointestinal diseases and about 5 × 107 cases of severe respiratory diseases are caused by swimming in wastewater polluted waters[71]. Bacillus amyloliquefaciens ST34 and Pseudomonas aeruginosa ST5 are biosurfactant-producing bacteria that have been isolated from wastewaters, producing surfactin and rhamnolipids with antimicrobial activity and a broad spectrum of other pathogens including drug-resistant Staphylococcus aureus, Escherichia coli strains and Candida albicans[72]. This indicates that they could have an important role in the initial biological treatment stages of wastewater. Poorly treated sewage from hospitals and quarantine facilities can be a potential source of the spread of pathogens. Wastewater treatment was shown to be enhanced when a lipopeptide was applied to a lignocellulosic biocomposite. This resulted in a boost of the adsorption characteristics of the biocomposite through an increase in its stability, roughness, sharpness, and roundness[70]. By trapping compounds such as heavy metals and xenobiotics, and reducing the BOD in water treatment process, this contributes to a decrease in microbial pathogen populations. Various combinations of biosurfactant-producing bacteria have been isolated from wastewater. A combination of biosurfactant-producing bacteria with very broad spectrum activity could be considered as additional treatment options in wastewater facilities.
3.3.2. Vector Control
Infectious disease transmission can be from person-to-person or through a biological intermediate organism called a vector. Vectors to most important infectious diseases are insects which are commonly found in our environment. Important viral diseases such as dengue, chikungunya and Zika have all been causes of serious outbreaks in many regions worldwide. Their vector, Aedes sp. mosquito, is in contact with over half of the world’s population[73]. Protozoans are thought to have an unlimited pandemic potential; they are the only species which vector infectious diseases that have caused the extinction of a mammalian species. This was observed with Trypanosoma lewisi, a vector-borne disease that made the Christmas Island rat (Rattus macleari) in Australia extinct around the early 20th century[74] . Furthermore, the malaria-causing protozoan Plasmodium was also thought to have killed 50% of all humans that existed on earth[73]. Trypansomiasis and other vector-borne pathogens can be confined to a geographical region because of the limitation of movement of the vector pathogen and the inability to survive in various earth climatic regions[73]. Nevertheless, their impact on human health cannot be overlooked.
Biosurfactant-containing biopesticides are biodegradable, show lower resistance in insect populations, have higher selectivity and biological safety compared to non-target species, in addition to being highly effective at lower concentrations[75]. Biosurfactant lipopeptides have been used as bioactive components in biopesticide formulations containing Bacillus thuringiensis. These formulations are lethal to the pupal and larval stages of insect vectors, with mosquitocidal activity against Aedes aegypti, the vector of dengue fever[76], Anopheles stephensi, the primary mosquito vector of malaria in India[77], and Culex quiniquefasciatus, a vector for arboviruses and avian malaria[78]. ZnO nanoparticles synthesized with B. licheniformis EPS showed toxicity against the larvae of malaria and Zika virus vectors Anopheles stephensi and Aedes aegypti, with high biocompatibility and non-toxicity demonstrated on hemolysis potential tests[79]. Insect control through outdoor and indoor spraying is an important control approach in disease control programs in regions affected by vector-borne diseases. Conventional pesticides such as DDT have issues of environmental toxicity and developing insect resistance. Biosurfactants could be the solution to more effective and environmentally friendly biopesticides use.
3.4. Hygiene and Personal Protective Equipment
Outbreak prevention and management requires the application of integrated approaches in combination with therapeutics. For vector-borne diseases, environmental control is certainly important. In the case of infectious diseases that can be transmitted from person-to-person directly or indirectly through fomites, hygiene and personal protection is also an important factor to consider. The environmental and biological safety of the products to be used is not to be overlooked. Protective equipment such as masks are widely recommended to limit the spread of COVID-19, although gradually constitute an environmental problem especially with single usage masks that are discarded everywhere. Although conventional facemasks offer protection against pathogens in aerosols by acting as a barrier, they are not lethal to the pathogens. This implies there is still a risk of infection which is the reason for their one-time usage. Masks from biodegradable silk or biodegradable polymers conjugated with cotton can facilitate degradability and user-friendliness. Biosurfactants are biodegradable and less biologically toxic; masks with additional silk layers and impregnated with biosurfactant material can offer both effective filtering and lethality to pathogens[1].
Biosurfactants on the surface of materials can confer many benefits. The antimicrobial and antiadhesive and antibiofilm properties of biosurfactants have been reported[80]. Trehalose lipids specifically were investigated using modified polystyrene and silicone surfaces and shown to inhibition colonization on polystyrene and silicone surfaces[57][81]. Biosurfactants produced by Lactobacillus rhamnosus and Lactobacillus jensenii also showed antimicrobial, antiadhesive and antibiofilm activity against MDR bacteriaAcinetobacter baumannii and E. coli on surfaces, at 25–100 mg/mL, inhibition of biofilm formation at 25–50 mg/mL, and dispersed already-formed biofilms of S. aureus and A. baumannii at 50–100 mg/mL[82].
Antimicrobial activity has also been observed with polyvinyl alcohol, as well as a polyvinyl alcohol–biosurfactant mixture in plastic and glassware[83]. This can enable the development of re-usable consumables and disinfectants for hospital equipment. Biosurfactants with higher molecular mass are generally considered to be better emulsifiers, forming long-lasting stable emulsions which are highly desirable properties in cleaning[84]. For detergency and cleaning, the amphiphilic nature of biosurfactants allows them to bind simultaneously to hydrophobic moieties of microbes with the fatty acid chains and water with their hydrophilic moieties. This results in emulsification that removes dirt from the surface followed by solubilization into small droplets[29].
Nosocomial infections could cause hospital-bound outbreaks within the hospital environment. Hospital areas are often characterized by rapid recontamination of disinfected areas, multi-drug resistance development potential, and limited time for the action of cleaning agents. Additionally, the use of synthetic surfactants does not prevent reinfection, can lead to increased pollution, and may provoke chemical sensitivity reactions in patients. Surfactant-based cleaning agents used in hospital environment showed promising results as alternatives to chemical-based surfactants, reducing >80% of S. aureus, Pseudomonas sp., Candida sp. and coliforms with bio-stabilization of the microbial load over time[85]. Additionally, biosurfactants isolated from psychrophiles can be used effectively at cold temperatures. Viral particles and pathogens often become inactive in cold conditions but reactivate when conditions become favorable. Most additives are also inactive at cold temperatures, which may make such environments a potential reservoir for pathogens. A more environmentally friendly and bactericidal effect can be obtained using biosurfactants in washing or storage requiring cold temperatures[8].
Furthermore, biosurfactants are structurally versatile and can be combined with enzymes in detergent formulations as well as to possibly make the formulation fully renewable[86]. Common household cleaning products and detergents contain typically 15–40% surfactants, which can often cause skin irritations as compared to biosurfactants which cause little or no irritation[29]. Initial commercialization of biosurfactant-based products for household and personal care are already seen with sophorolipids produced by Evonik[87]. Skin tenderness, biodegradability, good cleaning, and environmental friendliness are amongst the properties reported for these products[87]. Chemical companies are partnering to produce a new brand of renewable and biodegradable biobased household cleaning products, based on rhamnolipids currently used in Chile[88]. The applications of biosurfactants in cleaning are many and fully unexplored. As next-generation green molecules, they can play a role in limiting the spread of both known and unknown pathogens during outbreaks through effective hygiene.
3.5. Food Outbreak Control
There are suggestions of the possible transmission of COVID-19 through frozen foods. Viable SARS-CoV-2 pathogens have been found in frozen food storage areas, food, and its packaging[89]. Despite evidence that cold temperatures can prolong the shelf life of most pathogens; this has not received much attention in the case of COVID-19. As a matter of fact, there is no conclusive information available regarding the duration of COVID-19 persistence in different environments and surfaces[90][91]. However, more specific studies have shown that SARS-CoV-2 can survive at 4 to −80 °C on refrigerated foods such as meat, fish, poultry, or pork for 14–21 days. News reports from the Chinese CDC also mentioned findings of traces of COVID-19 on frozen cod packaging[89]. There could be an even wider threat through retail cold stores, where people travel to purchase daily groceries and then disperse to different regions through transportation. Although the pathogenicity and infectivity of the virus at those conditions have not yet been ascertained, it remains a possible route for spread and propagation of the virus[92].
Other known serious food-borne outbreak causative pathogens include Norovirus,Clostridium perfringens, Salmonella, Staphylococcus aureus and Campylobacter[93]. Biosurfactants could provide another opportunity for the interruption of infectious disease spread through food, mainly due to their previously mentioned relevant applications in packaging and their potential use in cold environments and as additives to the food industry[94]. In addition, as a subsidiary use to food industry applications and due to biosurfactants’ abilities to act as effective detergents with the added benefit of antimicrobial activity, their use as surface cleaning products could be highly advantageous.
4. Conclusions and Future Perspectives
Pandemic preparedness and management are integrated approaches involving a wide range of measures that can be applied simultaneously to build resilience against the causative agent. Recognizing the characteristics and potential for certain microorganisms that can cause a pandemic and developing pipelines for drugs, vaccines, and other pharmaceuticals against these potential agents for both broad-spectrum and specific pathogens will be of significant benefit to the development of resilience against these agents in the future. Our environment is also a major transmission vehicle for various pathogens and agents capable of disease outbreaks. We need to understand the role it plays in these events, and develop warning and response systems while maintaining a healthy environment. Exponential disease spread through direct and indirect contact with people can be limited using sustainable biosurfactants in hygiene and cleaning product formulations. There are many opportunities for biosurfactants to be applied as direct agents on virulent microbes as well as in other relevant interventions. Future technologies such as nanobiotechnology and various drone applications are also seen to be compatible with biosurfactants. For the disinfection or biopesticide spraying of large surface areas, drones could be used to deploy biosurfactant-based products, or identify nanoparticles for laboratory diagnostics. This could be highly useful in outbreak emergencies. Although biosurfactants are increasingly showing innovative applications in various areas, being environmentally friendly and often more effective than synthetic surfactants, production is still relatively uncompetitive. More research directed towards reducing the cost of production, development of applications in unexplored areas, and to explore the effects of specific biosurfactants on specific pathogens to provide more conclusive evidence for further applications.
This entry is adapted from the peer-reviewed paper 10.3390/app11010334
References
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444.
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508.
- Çakmak, H.; Güngörmedi, G.; Dikmen, G.; Çelik, P.A.; Çabuk, A. The true methodology for rhamnolipid: Various solvents affect rhamnolipid characteristics. Eur. J. Lipid Sci. Technol. 2017, 119, 1700002.
- Marchant, R.; Banat, I.M. Microbial biosurfactants: Challenges and opportunities for future exploitation. Trends Biotechnol. 2012, 30, 558–565.
- Sobrinho, H.; Luna, J.M.; Rufino, R.D.; Porto, A.; Sarubbo, L.A. Biosurfactants: Classification, properties and environmental applications. Recent Dev. Biotechnol. 2013, 11, 1–29.
- Ben Ayed, H.; Jridi, M.; Maalej, H.; Nasri, M.; Hmidet, N. Characterization and stability of biosurfactant produced by Bacillus mojavensis A21 and its application in enhancing solubility of hydrocarbon. J. Chem. Technol. Biotechnol. 2014, 89, 1007–1014.
- Perfumo, A.; Banat, I.M.; Marchant, R. Going Green and Cold: Biosurfactants from Low-Temperature Environments to Biotechnology Applications. Trends Biotechnol. 2018, 36, 277–289.
- Lima, T.M.S.; Procópio, L.C.; Brandão, F.D.; Carvalho, A.M.X.; Tótola, M.R.; Borges, A.C. Biodegradability of bacterial surfactants. Biodegradation 2011, 22, 585–592.
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Fayaz, F.; Alara, O.R. Biosurfactants—A new frontier for social and environmental safety: A mini review. Biotechnol. Res. Innov. 2018, 2, 81–90.
- Hirata, Y.; Ryu, M.; Oda, Y.; Igarashi, K.; Nagatsuka, A.; Furuta, T.; Sugiura, M. Novel characteristics of sophorolipids, yeast glycolipid biosurfactants, as biodegradable low-foaming surfactants. J. Biosci. Bioeng. 2009, 108, 142–146.
- Liu, J.-F.; Mbadinga, S.M.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Chemical structure, property and potential applications of biosurfactants produced by Bacillus subtilis in petroleum recovery and spill mitigation. Int. J. Mol. Sci. 2015, 16, 4814–4837.
- Toptaş, Y.; Çelikdemir, M.; Tuncer, C.; Şahin, Y.B.; Çelik, P.A.; Burnak, N.; Çabuk, A.; Bütün, V. Optimization of a biosurfactant production from bacteria isolated from soil and characterization of the surfactant. Turk. J. Biochem. 2016, 41.
- Shoeb, E.; Akhlaq, F.; Badar, U.; Akhter, J.; Imtiaz, S. Classification and industrial applications of biosurfactants. Acad. Res. Int. 2013, 4, 243–252.
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2014, 5.
- Henkel, M.; Müller, M.M.; Kügler, J.H.; Lovaglio, R.B.; Contiero, J.; Syldatk, C.; Hausmann, R. Rhamnolipids as biosurfactants from renewable resources: Concepts for next-generation rhamnolipid production. Process. Biochem. 2012, 47, 1207–1219.
- Naughton, P.J.; Marchant, R.; Naughton, V.; Banat, I.M. Microbial Biosurfactants: Current trends and applications in Agricultural and Biomedical industries. J. Appl. Microbiol. 2019, 127, 12–28.
- Markets and Markets Biosurfactants Market Analysis Recent Market Developments Industry Forecast to 2016–2022. Available online: https://www.marketsandmarkets.com/Market-Reports/biosurfactant-market-163644922.html?gclid=CjwKCAiAjrXxBRAPEiwAiM3DQmdr778lPDUjK3XChKXZDoTAKDlQHs7eTX44QDmAHOOo_t91YemOSRoC0n8QAvD_BwE (accessed on 26 January 2020).
- WHO. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 24 October 2020).
- IPBES. Media Release: IPBES #PandemicsReport: Escaping the “Era of Pandemics” IPBES. Available online: https://ipbes.net/pandemics (accessed on 2 November 2020).
- WHO. WHO Disease outbreaks. Available online: http://www.who.int/emergencies/diseases/en/ (accessed on 24 October 2020).
- Elshikh, M.; Marchant, R.; Banat, I.M. Biosurfactants: Promising bioactive molecules for oral-related health applications. FEMS Microbiol. Lett. 2016, 363.
- Cameotra, S.S.; Makkar, R.S. Recent applications of biosurfactants as biological and immunological molecules. Curr. Opin. Microbiol. 2004, 7, 262–266.
- Gudina, E.J.; Rangarajan, V.; Sen, R.; Rodrigues, L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013, 34, 667–675.
- Rawat, G.; Dhasmana, A.; Kumar, V. Biosurfactants: The next generation biomolecules for diverse applications. Environ. Sustain. 2020.
- Mandana Ohadi; Arash Shahravan; Negar Dehghannoudeh; Touba Eslaminejad; Ibrahim M. Banat; Gholamreza Dehghannoudeh; Potential Use of Microbial Surfactant in Microemulsion Drug Delivery System: A Systematic Review. Drug Design, Development and Therapy 2020, 14, 541-550, 10.2147/dddt.s232325.
- Eduardo J. Gudiña; Vivek Rangarajan; RamKrishna Sen; Lígia R. Rodrigues; Potential therapeutic applications of biosurfactants. Trends in Pharmacological Sciences 2013, 34, 667-675, 10.1016/j.tips.2013.10.002.
- Mandana Ohadi; Bagher Amir-Heida; Mohammad Hassan Moshafi; Ali Mirparizi; Mohammadzaman Basir; Gholamreza Dehghan-No; Encapsulation of Biosurfactant-Producing Bacillus licheniformis (PTCC 1320) in Alginate Beads. Biotechnology(Faisalabad) 2014, 13, 239-244, 10.3923/biotech.2014.239.244.
- Vincent Faivre; Véronique Rosilio; Interest of glycolipids in drug delivery: from physicochemical properties to drug targeting. Expert Opinion on Drug Delivery 2010, 7, 1031-1048, 10.1517/17425247.2010.511172.
- Matthew L. Smith; Stefano Gandolfi; Philippa M. Coshall; Pattanathu K. S. M. Rahman; Biosurfactants: A Covid-19 Perspective. Frontiers in Microbiology 2020, 11, 1341, 10.3389/fmicb.2020.01341.
- Prakash Palanisamy; Biosurfactant mediated synthesis of NiO nanorods. Materials Letters 2008, 62, 743-746, 10.1016/j.matlet.2007.06.053.
- Prakash Palanisamy; Ashok M. Raichur; Synthesis of spherical NiO nanoparticles through a novel biosurfactant mediated emulsion technique. Materials Science and Engineering: C 2009, 29, 199-204, 10.1016/j.msec.2008.06.008.
- Charles B.B. Farias; Aline Ferreira Silva; Raquel Diniz Rufino; Juliana Moura Luna; José Edson Gomes Souza; Leonie Asfora Sarubbo; Synthesis of silver nanoparticles using a biosurfactant produced in low-cost medium as stabilizing agent. Electronic Journal of Biotechnology 2014, 17, 122-125, 10.1016/j.ejbt.2014.04.003.
- G. Seghal Kiran; A. Sabu; Joseph Selvin; Synthesis of silver nanoparticles by glycolipid biosurfactant produced from marine Brevibacterium casei MSA19. Journal of Biotechnology 2010, 148, 221-225, 10.1016/j.jbiotec.2010.06.012.
- C Ganesh Kumar; Suman Kumar Mamidyala; Biswanath Das; B Sridhar; G Sarala Devi; Mallampalli Srilakshmi Karuna; Synthesis of biosurfactant-based silver nanoparticles with purified rhamnolipids isolated from Pseudomonas aeruginosa BS-161R.. Journal of Microbiology and Biotechnology 2010, 20, 1061-1068, .
- Yingwei Xie; Ruqiang Ye; Honglai Liu; Synthesis of silver nanoparticles in reverse micelles stabilized by natural biosurfactant. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2006, 279, 175-178, 10.1016/j.colsurfa.2005.12.056.
- Mohammad Asaduzzaman Chowdhury; Nayem Hossain; Mohammod Abul Kashem; Abdus Shahid; Ashraful Alam; Immune response in COVID-19: A review. Journal of Infection and Public Health 2020, 13, 1619-1629, 10.1016/j.jiph.2020.07.001.
- Mehfuz Ezaman; Istvan Toth; Immunostimulation by Synthetic Lipopeptide-Based Vaccine Candidates: Structure-Activity Relationships. Frontiers in Immunology 2013, 4, 318, 10.3389/fimmu.2013.00318.
- Karl Deres; Hansj; [Ouml]; Rg Schild; Karl-Heinz Wiesmüller; In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature 1989, 342, 561-564, 10.1038/342561a0.
- Brenda Kischkel; Suélen A. Rossi; Samuel R. Junior Santos; Joshua D. Nosanchuk; Luiz R. Travassos; Carlos Pelleschi Taborda; Therapies and Vaccines Based on Nanoparticles for the Treatment of Systemic Fungal Infections. Frontiers in Cellular and Infection Microbiology 2020, 10, 463, 10.3389/fcimb.2020.00463.
- Henrik Garoff; Roger Hewson; Dirk-Jan E. Opstelten; Virus Maturation by Budding. Microbiology and Molecular Biology Reviews 1998, 62, 1171-1190, .
- Luis F. García; Immune Response, Inflammation, and the Clinical Spectrum of COVID-19. Frontiers in Immunology 2020, 11, 1441, 10.3389/fimmu.2020.01441.
- Mohana Devi Subramaniam; Dhivya Venkatesan; Mahalaxmi Iyer; SarathBabu Subbarayan; Vivekanandhan Govindasami; Ayan Roy; Arul Narayanasamy; Siva Kamalakannan; Abilash Valsala Gopalakrishnan; Raviminickam Thangarasu; et al. Biosurfactants and anti-inflammatory activity: A potential new approach towards COVID-19. Current Opinion in Environmental Science & Health 2020, 17, 72-81, 10.1016/j.coesh.2020.09.002.
- Nicholas D. Weber; Martine Aubert; Chung H. Dang; Daniel Stone; Keith R. Jerome; DNA cleavage enzymes for treatment of persistent viral infections: recent advances and the pathway forward.. Virology 2014, 454, 353-61, 10.1016/j.virol.2013.12.037.
- Yunshan Liang; Xingzhong Yuan; Guangming Zeng; Hua Zhong; Hui Li; Weiwei Wang; Effects of surfactants on enzyme-containing reversed micellar system. Science China Chemistry 2011, 54, 715-723, 10.1007/s11426-011-4266-2.
- Hamidreza Hajfarajollah; Parisa Eslami; Babak Mokhtarani; Kambiz Akbari Noghabi; Biosurfactants from probiotic bacteria: A review. Biotechnology and Applied Biochemistry 2018, 65, 768-783, 10.1002/bab.1686.
- Eduardo J. Gudiña; José A. Teixeira; Lígia R. Rodrigues; Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloids and Surfaces B: Biointerfaces 2010, 76, 298-304, 10.1016/j.colsurfb.2009.11.008.
- P. Saravanakumari; K. Mani; Structural characterization of a novel xylolipid biosurfactant from Lactococcus lactis and analysis of antibacterial activity against multi-drug resistant pathogens. Bioresource Technology 2010, 101, 8851-8854, 10.1016/j.biortech.2010.06.104.
- Deepansh Sharma; Baljeet Singh Saharan; Functional characterization of biomedical potential of biosurfactant produced by Lactobacillus helveticus. Biotechnology Reports 2016, 11, 27-35, 10.1016/j.btre.2016.05.001.
- Muthu Irulappan Sriram; Kalimuthu Kalishwaralal; Venkataraman Deepak; Raja Gracerosepat; Kandasamy Srisakthi; Sangiliyandi Gurunathan; Biofilm inhibition and antimicrobial action of lipopeptide biosurfactant produced by heavy metal tolerant strain Bacillus cereus NK1. Colloids and Surfaces B: Biointerfaces 2011, 85, 174-181, 10.1016/j.colsurfb.2011.02.026.
- Eduardo J. Gudiña; V. Rocha; J. A. Teixeira; L. R. Rodrigues; Antimicrobial and antiadhesive properties of a biosurfactant isolated fromLactobacillus paracaseissp.paracaseiA20. Letters in Applied Microbiology 2010, 50, 419-424, 10.1111/j.1472-765x.2010.02818.x.
- Deepansh Sharma; Baljeet Singh Saharan; Simultaneous Production of Biosurfactants and Bacteriocins by ProbioticLactobacillus caseiMRTL3. International Journal of Microbiology 2014, 2014, 1-7, 10.1155/2014/698713.
- Surekha K. Satputea; Nishigandha S. Mone; Parijat Das; Ibrahim M. Banat; Arun G. Banpurkar; Inhibition of pathogenic bacterial biofilms on PDMS based implants by L. acidophilus derived biosurfactant. BMC Microbiology 2019, 19, 1-15, 10.1186/s12866-019-1412-z.
- Surekha K. Satputea; Nishigandha S. Mone; Parijat Das; Arun G. Banpurkar; Ibrahim M. Banat; Lactobacillus acidophilus Derived Biosurfactant as a Biofilm Inhibitor: A Promising Investigation Using Microfluidic Approach. Applied Sciences 2018, 8, 1555, 10.3390/app8091555.
- Liisa Lehtoranta; A. Pitkäranta; R. Korpela; Probiotics in respiratory virus infections. European Journal of Clinical Microbiology & Infectious Diseases 2014, 33, 1289-1302, 10.1007/s10096-014-2086-y.
- Yang, Jaemoon. Deuterium; Elsevier: Amsterdam, The Netherlands, 2016; pp. 116.
- Thomas J. Smyth; Amedea Perfumo; Roger Marchant; Ibrahim M. Banat; Minglei Chen; Robert K. Thomas; Jeff Penfold; Paul S. Stevenson; Neil J. Parry; Directed microbial biosynthesis of deuterated biosurfactants and potential future application to other bioactive molecules. Applied Microbiology and Biotechnology 2010, 87, 1347-1354, 10.1007/s00253-010-2592-5.
- Tomasz Janek; Anna Krasowska; Żaneta Czyżnikowska; Marcin Łukaszewicz; Trehalose Lipid Biosurfactant Reduces Adhesion of Microbial Pathogens to Polystyrene and Silicone Surfaces: An Experimental and Computational Approach. Frontiers in Microbiology 2018, 9, 2441, 10.3389/fmicb.2018.02441.
- Abulaziz Juma; Patrick Lemoine; Alistair B. J. Simpson; Jason Murray; Barry M. G. O’Hagan; Patrick J. Naughton; James G. Dooley; Ibrahim M. Banat; Microscopic Investigation of the Combined Use of Antibiotics and Biosurfactants on Methicillin Resistant Staphylococcus aureus. Frontiers in Microbiology 2020, 11, 1477, 10.3389/fmicb.2020.01477.
- Benjamin D. Brooks; Amanda E. Brooks; Therapeutic strategies to combat antibiotic resistance. Advanced Drug Delivery Reviews 2014, 78, 14-27, 10.1016/j.addr.2014.10.027.
- Yuan Liu; Dr. Shuangyang Ding; Richard Dietrich; Dr. Erwin Märtlbauer; Kui Zhu; A Biosurfactant‐Inspired Heptapeptide with Improved Specificity to Kill MRSA. Angewandte Chemie International Edition 2017, 56, 1486-1490, 10.1002/anie.201609277.
- Dirk Vollenbroich; Muhsin Özel; Joachim Vater; Roza Maria Kamp; Georg Pauli; Mechanism of Inactivation of Enveloped Viruses by the Biosurfactant Surfactin fromBacillus subtilis. Biologicals 1997, 25, 289-297, 10.1006/biol.1997.0099.
- Madiha Basit; Muhammad Hidayat Rasool; Syed Ali Raza Naqvi; Muhammad Waseem; Bilal Aslam; Biosurfactants production potential of native strains of Bacillus cereus and their antimicrobial, cytotoxic and antioxidant activities.. pakistan journal of pharmaceutical sciences 2018, 31, 251-256, .
- Lvfeng Yuan; Shuai Zhang; Jie Peng; Yuchen Li; Qian Yang; Synthetic surfactin analogues have improved anti-PEDV properties. PLoS ONE 2019, 14, e0215227, 10.1371/journal.pone.0215227.
- Lvfeng Yuan; Shuai Zhang; Yongheng Wang; Yuchen Li; Xiaoqing Wang; Qian Yang; Surfactin Inhibits Membrane Fusion during Invasion of Epithelial Cells by Enveloped Viruses. Journal of Virology 2018, 92, JVI.00809-18, 10.1128/jvi.00809-18.
- Grażyna Anna Płaza; Joanna Chojniak; Ibrahim M. Banat; Biosurfactant Mediated Biosynthesis of Selected Metallic Nanoparticles. International Journal of Molecular Sciences 2014, 15, 13720-13737, 10.3390/ijms150813720.
- 67. Katariya, H.B. the concept of microbubble as a drug delivery system: An overview. Int. J. Pharm. Sci. Res. 2012, 3, 3058–3063
- Qingyi Xu; Mitsutoshi Nakajima; Zengshe Liu; Takeo Shiina; Biosurfactants for Microbubble Preparation and Application. International Journal of Molecular Sciences 2011, 12, 462-475, 10.3390/ijms12010462.
- Khaled Al Huraimel; Mohamed Alhosani; Shabana Kunhabdulla; Mohammed Hashem Stietiya; SARS-CoV-2 in the environment: Modes of transmission, early detection and potential role of pollutions. Science of The Total Environment 2020, 744, 140946-140946, 10.1016/j.scitotenv.2020.140946.
- I. Michael-Kordatou; P. Karaolia; Despo Fatta-Kassinos; Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification. Journal of Environmental Chemical Engineering 2020, 8, 104306-104306, 10.1016/j.jece.2020.104306.
- M. Perez-Ameneiro; Xanel Vecino; Jose M. Cruz; Ana Belén Moldes; Wastewater treatment enhancement by applying a lipopeptide biosurfactant to a lignocellulosic biocomposite. Carbohydrate Polymers 2015, 131, 186-196, 10.1016/j.carbpol.2015.05.075.
- Hillel Shuval; Estimating the global burden of thalassogenic diseases: human infectious diseases caused by wastewater pollution of the marine environment. Journal of Water and Health 2003, 1, 53-64, 10.2166/wh.2003.0007.
- Thando Ndlovu; Sehaam Khan; Wesaal Khan; Distribution and diversity of biosurfactant-producing bacteria in a wastewater treatment plant. Environmental Science and Pollution Research 2016, 23, 9993-10004, 10.1007/s11356-016-6249-5.
- Amesh A. Adalja; Matthew Watson; Eric S. Toner; Anita Cicero; Thomas V. Inglesby; Characteristics of Microbes Most Likely to Cause Pandemics and Global Catastrophes. Current Topics in Microbiology and Immunology 2019, 424, 1-20, 10.1007/82_2019_176.
- Kelly B. Wyatt; Paula F. Campos; M. Thomas P. Gilbert; Sergios-Orestis Kolokotronis; Wayne L. Hynes; Rob DeSalle; Peter Daszak; Ross D. E. Macphee; Alex D. Greenwood; Historical Mammal Extinction on Christmas Island (Indian Ocean) Correlates with Introduced Infectious Disease. PLoS ONE 2008, 3, e3602, 10.1371/journal.pone.0003602.
- Mnif Inès; Dhouha Ghribi; Potential of bacterial derived biopesticides in pest management. Crop Protection 2015, 77, 52-64, 10.1016/j.cropro.2015.07.017.
- A.M. Manonmani; I. Geetha; S. Bhuvaneswari; Enhanced production of mosquitocidal cyclic lipopeptide from Bacillus subtilis subsp. subtilis. Indian Journal of Medical Research 2011, 134, 476-482, .
- I. Geetha; A.M. Manonmani; K. P. Paily; Identification and characterization of a mosquito pupicidal metabolite of a Bacillus subtilis subsp. subtilis strain. Applied Microbiology and Biotechnology 2010, 86, 1737-1744, 10.1007/s00253-010-2449-y.
- Kishore Das; Ashis K. Mukherjee; Assessment of mosquito larvicidal potency of cyclic lipopeptides produced by Bacillus subtilis strains. Acta Tropica 2006, 97, 168-173, 10.1016/j.actatropica.2005.10.002.
- Muthukumar Abinaya; Baskaralingam Vaseeharan; Mani Divya; Aruna Sharmili; Marimuthu Govindarajan; Naiyf S. Alharbi; Shine Kadaikunnan; Jamal M. Khaled; Giovanni Benelli; Bacterial exopolysaccharide (EPS)-coated ZnO nanoparticles showed high antibiofilm activity and larvicidal toxicity against malaria and Zika virus vectors. Journal of Trace Elements in Medicine and Biology 2018, 45, 93-103, 10.1016/j.jtemb.2017.10.002.
- Ibrahim M. Banat; Mayri A. Díaz De Rienzo; Gerry A. Quinn; Microbial biofilms: biosurfactants as antibiofilm agents. Applied Microbiology and Biotechnology 2014, 98, 9915-9929, 10.1007/s00253-014-6169-6.
- C. Ceresa; L. Fracchia; M. Williams; Ibrahim M. Banat; Mayri A. Diaz De Rienzo; The effect of sophorolipids against microbial biofilms on medical-grade silicone. Journal of Biotechnology 2020, 309, 34-43, 10.1016/j.jbiotec.2019.12.019.
- Karthik Sambanthamoorthy; Xiaorong Feng; Ruchi Patel; Sneha Patel; Chrysanthi Paranavitana; Antimicrobial and antibiofilm potential of biosurfactants isolated from lactobacilli against multi-drug-resistant pathogens. BMC Microbiology 2014, 14, 197-197, 10.1186/1471-2180-14-197.
- 84. Salman, J.A.S.; Al Kadhemy, M.; Jaleel, M.; Abdal, A. Effect of PVA, PVA/biosurfactant on some pathogenic bacteria in glass and plastic plates. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 301–309.
- Marcia Nitschke; S.G.V.A.O. Costa; Biosurfactants in food industry. Trends in Food Science & Technology 2007, 18, 252-259, 10.1016/j.tifs.2007.01.002.
- 86. Vandini, A.; Frabetti, A.; Antonioli, P.; Platano, D.; Branchini, A.; Camerada, M.; Lanzoni, L.; Balboni, P.; Mazzacane, S.; Reduction of the Microbiological Load on Hospital Surfaces Through Probiotic-Based Cleaning Procedures: A New Strategy to Control Nosocomial Infections. Journal of Microbiology & Experimentation 2014, 1, 27, 10.15406/jmen.2014.01.00027.
- Sagheer A. Onaizi; Lizhong He; A. P. J. Middelberg; Rapid screening of surfactant and biosurfactant surface cleaning performance. Colloids and Surfaces B: Biointerfaces 2009, 72, 68-74, 10.1016/j.colsurfb.2009.03.015.
- Focus on Surfactants; Evonik commercializes biosurfactants. Focus on Surfactants 2016, 2016, 3-4, 10.1016/j.fos.2016.07.046.
- Unilever and Evonik Partner to Launch Green Cleaning Ingredient . Unilever.com. Retrieved 2021-1-27
- Live Coronavirus Found on Frozen Food Packaging in China . The Guardian. Retrieved 2021-1-27
- Luca Fiorillo; Gabriele Cervino; Marco Matarese; Cesare D’Amico; Giovanni Surace; Valeria Paduano; Maria Teresa Fiorillo; Antonio Moschella; Alessia La Bruna; Giovanni Luca Romano; et al. COVID-19 Surface Persistence: A Recent Data Summary and Its Importance for Medical and Dental Settings. International Journal of Environmental Research and Public Health 2020, 17, 3132, 10.3390/ijerph17093132.
- Gabriele Cervino; Luca Fiorillo; Giovanni Surace; Valeria Paduano; Maria Teresa Fiorillo; Rosa De Stefano; R. Laudicella; Sergio Baldari; Michele Gaeta; Marco Cicciù; et al. SARS-CoV-2 Persistence: Data Summary up to Q2 2020. Data 2020, 5, 81, 10.3390/data5030081.
- Jie Han; Xue Zhang; Shanshan He; Puqi Jia; Can the coronavirus disease be transmitted from food? A review of evidence, risks, policies and knowledge gaps. Environmental Chemistry Letters 2020, , , 10.1007/s10311-020-01101-x.
- Foodborne Illnesses and Germs . CDC. Retrieved 2021-1-27
- Jenyffer Medeiros Campos; Tânia Lúcia Montenegro Stamford; Leonie Asfora Sarubbo; Juliana Moura De Luna; Raquel Diniz Rufino; Ibrahim M. Banat; Microbial biosurfactants as additives for food industries. Biotechnology Progress 2013, 29, 1097-1108, 10.1002/btpr.1796.
- Jenyffer Medeiros Campos; Tânia Lúcia Montenegro Stamford; Leonie Asfora Sarubbo; Juliana Moura De Luna; Raquel Diniz Rufino; Ibrahim M. Banat; Microbial biosurfactants as additives for food industries. Biotechnology Progress 2013, 29, 1097-1108, 10.1002/btpr.1796.
