In addition to the edible kernel, the peanut seed consists of the woody outer shell and a paper-like substance that surrounds the kernel itself known as the testa or skin.
- peanut skins
- peanut testa
1. Introduction
The global production of peanuts is projected to be 47 metric tons for the 2020 crop year [1]. In addition to the edible kernel, the peanut seed consists of the woody outer shell and a paper-like substance that surrounds the kernel itself known as the testa or skin. For most peanut products, the skin is removed and discarded [2]. The skin removal is done by a process known as blanching, which subjects the shelled raw peanut kernels to mild dry heat treatment and mechanical abrasion. The skin portion represents approximately 3% of the total kernel mass, resulting in thousands of tons of this material being produced each year which has no real food value. It has some applications in animal feed but is limited by the bitter flavor and high levels of protein binding components, which have been identified as polyphenols [3][4][5]. It is these polyphenolic compounds that have proven to give value to peanut skins. Information on peanut skins has briefly been included in a recent review [6]. Readers are referred to another recent review for a more complete discussion of the types of phenolic compounds that are found in nuts, including peanuts [7].[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57]
2. Extraction of Peanut Skins
2.1. Compound Identification
The first reported attempts to chemically characterize peanut skins were concerned with their pigmentation. Off colors in isolated peanut protein were attributed to high molecular weight polyphenols or tannins leaching from the peanut skins during processing [8]. An early review of the protein-bound anthocyanins identified peanut skins as a source of those compounds [9]. Tannins were known for their metal-chelating properties and peanut skins were reported as an inexpensive source of these compounds that could be complexed and used to remove heavy metals from wastewater [10]. Removal of skins was also proposed as a method to reduce the aflatoxin content of peanuts [11]. This fungitoxicity led to research on the relationship of the content of these compounds with peanut maturity [12]. One of the first studies to try to elucidate the actual structure of the compounds investigated the possibility that peanut skin extracts might be a substitute for pine bark in preparing phenolic resins [13].
The early reports of extraction of peanut skins were concerned with removing compounds that caused discoloration of the kernels and were not concerned with protecting or recovering these compounds for further use [14]. In some cases, the solvents used are listed but the mixtures are not adequately described to determine how they have been used [15]. Extractions for identification of these compounds followed schemes that utilized the affinity of the hydroxyl groups present for polar solvents mixed with water [13]. Considering the compounds of interest would be similar to those of peanut hulls, methanol alone was used to prepare extracts of peanut skins that were tested as antioxidants for sunflower oil without determination of the identity of the compounds [16]. The findings that peanut skin extracts had antioxidant activity in a vegetable oil model led to studies to optimize the total phenolic content recovered using different solvent systems [17]. Table 1 lists the results from the solvent trials.
Table 1. Extraction percentages (wt %) of antioxidant components and phenolic total contents (mg/g) of the extracts from peanut skins using different solvents [17].
| Extract a | Extraction Percentage b | Phenolic Total Content b |
|---|---|---|
| ME | 17.9 (cd) ± 0.6 | 148.7 (d) ± 3.6 |
| EE | 18.5 (cd) ± 0.2 | 114.8 (c) ± 5.9 |
| KE | 19.4 (de) ± 0.6 | 61.4 (a) ± 1.4 |
| AE | 9.9 (a) ± 0.1 | 58.5 (a) ± 2.4 |
| dME | 17.1 (cd) ± 0.9 | 165.6 (a) ± 16.2 |
| dEE | 16.2 (c) ± 1.1 | 150.4 (d) ± 9.1 |
| dKE | 13.1 (b) ± 0.1 | 65.5 (a) ± 1.8 |
| dAE | 10.0 (a) ± 0.3 | 90.7 (b) ± 1.1 |
a Abbreviations: ME = Methanolic extract, EE = Ethanolic extract, KE = Acetonic extract, AE = Aqueous extract. The addition of the letter “d” signifies defatted peanut skins. b Means followed by the same letter within each column are not significantly different at α = 0.05.
Using a series of extractions, better isolation was possible for the identification of the actual compounds present in peanut skins [18]. Beginning with hot water, the bulk of the polar compounds was removed. The solution was passed through a non-polar polymer resin (HP20), and the hydroxylated compounds were then eluted with aqueous acetone (70%). The residue remaining after solvent removal was further purified by extraction with ethanol (95%). After solvent removal, the resulting residue was passed through a size-exclusion resin. Different fractions (12) were eluted from the resin using several combinations of aqueous acetone with and without the addition of acetone. With several purifications using silica gel, followed by a final separation using preparative High-Pressure Liquid Chromatography (HPLC), several fractions were produced that were finally recovered using recrystallization to allow for identification using Nuclear Magnetic Resonance Spectroscopy (NMR).
Peanut skins have been used in traditional Chinese medicine preparations to treat conditions such as chronic hemorrhages and bronchitis. To investigate these applications, the compounds extracted using aqueous solvent mixtures were identified as the A-type proanthocyanidins [18]. Some of the phenolic containing compounds isolated in this study were found to inhibit the activity of hyaluronidase, an enzyme which has been found to increase in the presence of certain cancers [19]. A follow-up study further isolated and purified the extracts [20]. From the NMR spectra, the alkaloids, 3-methoxy-3(3-indolyl)-propionic acid, 2-hydroxy-3-[3-(1-N-methyl)-indolyl]-propionic acid, and 2-methoxy-3-(3-indolyl)-propionic acid were identified. The authors reported that the first two compounds had never been isolated from a natural source. Other compounds identified were several flavonoid glycosides. Further research with this extraction technology allowed for the isolation and identification of several oligomeric proanthocyanidins [21]. The activity of the flavan-3-ols, catechin and epicatechin and their oligomers, and the proanthocyanidins has been the subject of research in cocoa sources and tea [22]. As those found in peanut skins are composed of the A linkage form compared to the B or C forms in those other sources, comparison studied have investigated the comparative activity. The difference in the 3-dimensional structure of the compounds due to the extra 2β-O7 linkage in the A-type found in peanut skins could affect their interaction with membrane phospholipids (Figure 1).
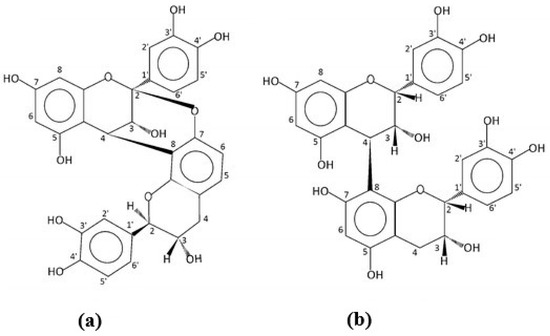
Figure 1. Structures of the procyanidin dimers. (a) The A-form found in peanut skins. (b) The B-form found in cocoa.
As this has proven to influence membrane fluidity and thus the ability of the compounds to interact with free radicals within the cell, the action of the different forms was examined [23]. A system using fluorescent probes that could be oxidized by peroxyl radicals that were induced by the assay on liposomes was used. The effectiveness was found to be dependent on the number of available flavanol monomers present, so no significant differences were seen between the types of dimers and trimers. The A-type dimer did have a different effect on the ordering of the core, with different rigidification indicating a different type of reaction with this form of the dimer. It has been proposed that the rigidity of the A-type due to the additional bond between the adjoining flavan-3-ols plays a role in their interaction with large biomolecules [24].
The color of peanut skins has been proven to have a relationship with the composition of the phenolic compound present [25][26][27][28][29]. In an examination of all the market types with skin colors from light brown to dark red, total flavonoids were not found to be as closely correlated to skin color as they were to growing location. The statistical relationship between the total flavonoids and the procyanidin content indicated that these compounds are the major flavonoids present. This indicates these compounds are more sensitive to stress conditions. The total phenols were more closely related to the hue of the skin color. Only the peanuts with the black seed coats were found to contain cyanidin-3-O-sambubioside both free and in glucosides [27]. Black seed coated peanuts were used to study the genetic control of peanut seed coat color [30]. In this report, the flavonoids present in white, red, and striped peanut skins in addition to those in the black peanut seed coats were extracted using 75% methanol in water acidified with 0.5% acetic acid. The extracts were filtered, and the flavonoids were identified and quantified using High-Pressure Liquid Chromatography-Mass Spectrometry-Time of Flight (HPLC-MS-TOF). Although different flavonoids and their glycosides were found to be unique to the different colored peanut skins, they were found to have the same biosynthetic pathways for anthocyanins but with different modifications. Individual flavonoids were isolated and identified from the skins of black seed-coated peanuts after extraction with acidified water followed by a partition into ethyl acetate [29]. The extracts were fractionated using Amberlite XAD-7HP resin to remove the most polar compounds followed by YMC® Gel ODS-AQHG resin to separate the hydrophilic ones and identified using High-Pressure Liquid Chromatography-Electrospray Ionization-Mass Spectrometry (HPLC-ESI-MS/MS) and NMR spectroscopy. Three unique flavonoids (quercetin-methylpentoside, quercetin-feruloyl-hexoside, quercetin-3-dihexoside) and four anthocyanins (cyanidin-3-o-sophoroside, cyanidin-3-o-sambubiside, cyandin-3-o-glucosylrutinoside, cyanidin-3-o-xylosylrutioside) were identified.
Using HPLC to determine the various compounds that compose peanut skins has advanced from initial reports of the oligomers [31]. Aqueous ethanol was the extractant for the analysis of peanut skins, and the method identified five phenolic acids (gallic acid, caffeic acid, p-coumaric acid, protocatechuic acid, ellagic acid), two stilbenes (piceid, resveratrol), and eight flavonoids (catechin, epicatechin, epigallocatechin, catechin gallate, epicatechin gallate, epigallocatechin gallate, procyanidin B2, quercetin). This report validated the methodology for the quantitation using the runner, Spanish, and Virginia market types. Concentrating specifically on peanut skins from the Virginia market type, a study compared the compounds extracted when either methanol, ethanol, acetone, or water at boiling temperature was used individually [32]. Using 100% acetone resulted in the highest amount of the smallest phenolic compounds. The study used HPLC-MSn to identify a range of compounds that were polymers of catechin and epicatechin with and without sugar moieties up to 9 catechins. These polymers were found mostly in the A form. An advanced study to determine the content and structural formation of the trimer and tetramer of the procyanidins used extraction with 30% methanol in water followed by 70% acetone in water [24]. The extracts were combined, and the solvent was evaporated to dryness. The dried material was dissolved in water and partitioned against ethyl acetate containing increasing amounts of aqueous ethanol (5%, 10%, 15%). The extracts were then loaded onto silica gel and rinsed with a series of solvents to purify and isolate the components. Identification was performed using NMR and electron circular dichroism spectroscopy (CD). The different polymers were tested for anti-inflammatory activity in a macrophage system and the tetramers were found to be the most effective with the dimers having little or no effect. This showed that biological systems could differentiate between the different forms of the procyanidins.
As studies became more sophisticated to examine the composition of peanut skins in order to determine the source of bioactivity, multistep extraction schemes were used to selectively isolate certain compounds. A series of methanol and water mixtures was used to remove specifically the A-type procyanidins [33]. Drawing on previous research, the extraction scheme was created [18][20][21]. Peanut skins were extracted sequentially with 20% aqueous methanol, 70% aqueous methanol, and then finally 70% aqueous acetone. Each fraction was concentrated and then partitioned into ethyl acetate to remove soluble saccharides. The fractions were then analyzed by HPLC-MS, which revealed the 20% methanol fraction contained mostly oligomers of the A-type procyanidins and the 70% acetone fraction contained larger polymers. The use of peanut skins as a specific source of compounds for isolation has been described. Peanut skin has been a source of resveratrol and was extracted using 20% ethanol in water [34]. Increasingly, previously unidentified compounds have been reported from peanut skin extractions. Researchers have described an A-type procyanidin (epicatechin-(2β→O→7,4β→8)-[catechin-(6→4β)]-epicatechin) that had not been reported before after extraction of peanut skins with 70% acetone in water, followed by fractionation on Sephadex LH-20 and elution with ethanol [35].
Research to differentiate between the classes of procyanidins present in peanut skins has used the technique of hydrogen/deuterium exchange (HDX) to elucidate the structural differences between isomers [36]. The technique was able to differentiate between the A-type procyanidins in peanut skin extracts containing up to three linkages. The extracts were prepared using 70% acetone in water containing 0.1% formic acid after defatting of the peanut skins. The filtered extracts were dried under nitrogen gas and then reconstituted in the deuterated mobile phase.
As a follow-up to a previous study of the composition of whole peanuts, the authors used the same extraction system of 80% methanol in water after defatting with hexane to compare peanut skins from two different peanut market types [37][38]. The analysis using HPLC-MS of the extracts found that the Valencia market type peanut skin extracts had higher levels of flavonols, quercetin, and its methylated analog, isorhamnetin.
One of the most complete studies of the phenolic type compounds in peanut skins used the extraction procedure developed for the analysis of grains to prepare samples for instrumental analysis [39]. The peanut skins analyzed were obtained after commercial blanching, which requires mild heat treatment. As heat treatment has been reported to liberate smaller phenolic compounds from larger polymers, this proved advantageous in allowing for a range of identifications [40]. Acidified water was used for the initial extraction followed by a partition into diethyl ether to capture the free phenolic compounds. A solvent exchange of methanol for the ether was performed before the analysis by HPLC-MS. From this, 88 individual phenolic type compounds were found, although some could only be identified by their class (Table 2). In addition, 60 proanthocyanidins were found, with most being of the A-type. The same group used the same extraction scheme with fractionation to determine the bound phenolics [41]. After the extraction and portioning of the free phenolics into diethyl ether, the aqueous phase was then base-hydrolyzed and acid-hydrolyzed to convert the ester derivatives to their carboxylic acid or flavonoid analogs. The analysis was then done with the same LC-MS system. An additional 78 compounds that existed as esters or glycosides were tentatively identified. The reader is referred to these publications for the listings of these compounds.
Table 2. Content of selected phenolics quantified in dry-blanched peanut skins (PS) by C18 Reverse Phase High-Pressure Liquid Chromatography (RP-HPLC) [39].
| Free Phenolic Compounds a | Content b (mg/100 g) |
|---|---|
| Protocatechuic acid | 3.43 ± 0.04 |
| p-Hydroxybenzoic acid | 1.03 ± 0.06 |
| Caftaric acid | 51 ± 0.12 |
| cis-Coutaric acid | 10.1 ± 0.52 |
| trans-Coutaric acid | 2.11 ± 0.08 |
| p-Coumaroyl-O-pentoside | 5.52 ± 0.23 |
| p-Coumaric acid | 0.53 ± 0.06 |
| Chicoric acid | 3.44 ± 0.12 |
| p-Coumaroylcaffeoyltartaric acid | 2.26 ± 0.13 |
| Chicoric acid | 3.12 ± 0.13 |
| di-p-Coumaroyltartaric acid | 13.8 ± 1.53 |
| p-Coumaroylsinapoyltartaric acid | 6.32 ± 0.94 |
| p-Coumaroylferuloyltartaric acid | 5.87 ± 0.71 |
| trans-Resveratrol | 0.36 ± 0.05 |
| Quercetin | 2.11 ± 0.27 |
| Isorhamnetin | 1.51 ± 0.02 |
| Diosmetin | 0.40 ± 0.01 |
a Caftaric acid and chicoric acids were quantified as caffeic acid equivalents; coutaric acids and other p-coumaroyl derivatives were quantified using p-coumaric acid equivalents; isorhamnetin and diosmetin were quantified using corresponding flavonoid aglycone equivalents. b Values are reported as means of triplicate analyses ± standard deviation. Findings are reported as mg respective phenolic/100-g dry weight (d.w.) of dry-blanched PS.
2.2. Extraction Optimization
The microwave-assisted extraction was another method used for peanut skins [42]. In this case, 30% ethanol in water was chosen as this had previously been proven to be the optimum for total phenolic extraction [43]. Surface response methodology was used to determine the power and time to extract the highest total phenols without decreasing the antioxidant effect. It was reported that 30 s at 90% microwave power produces the optimum product.
As the interest in recovering phenolic compounds from peanut skins increased, studies to optimize their recovery were done. Most of these studies concentrated on achieving the highest values using the Folin-Coicalteu assay rather than any defined class of polyphenols [44]. Factors such as particle size of the skin material, the proportion of solvent to the mass of skins, contact time with the skin material, maceration or shaking, and the number of extractions using 70% ethanol in water were evaluated. The efficiency of the extraction was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay for free radical scavenging power, which indicated that the method of agitation was not significant and that 10 minutes of extraction at 40 mL per 5 grams of peanut skins using 4 extractions was optimum. An optimization study examining proanthocyanidins from peanut skins used aqueous ethanol and ultrasonic extraction [45] (an aqueous solution of ethanol (55%) at an outlet power of 120 W at 35 °C). This resulted in the extraction of proanthocyanidins with a yield of 12.1%.
Water only was used to extract primarily the procyanidins that were then used to evaluate the potency to suppress allergenic response [46]. Further purification of the extracts was done with a reverse-phase resin to bind the polyphenols. Successive elutions were made with increasing concentrations of acetone in water and finally with 80% ethanol in water. The final eluate was then further purified using a size-exclusion resin, followed by a gel filtration resin and finally with silica gel to isolate specifically the procyanidin A-1 for further testing. When compared with the unpurified extract from the peanut skins, the isolated compounds were not as potent in suppressing immunoglobulin synthesis and regulating systemic T helper cytokine production.
Studies dedicated to the optimization of the extraction of phenolic compounds from peanut skins used response surface methodology and various chemical assays for comparisons [47][48]. Ethanol and methanol at concentrations of 30%, 60%, and 90% in water were compared with pure water and pure ethyl acetate. The total phenolics recovered (TPC) were optimized in ethanol at 30% (118 mg Gallic Equivalents per gram skins) and in methanol at 60% (112 mg Gallic Equivalents per gram skins). Pure water recovery was lower at 81 mg and ethyl acetate recovery was an order of magnitude lower. Using the Oxygen Radical Absorbance Capacity (ORAC) activity as the measure of antioxidant activity, the optimized alcohol extracts were similar (2050 μmol Trolox equivalents per gram for ethanol and 2149 μmol Trolox equivalents per gram for methanol). Pure water was less than half as effective. Varying the temperature of the extraction was investigated in this study. Increasing the temperature from 30 °C to 60 °C using 30% ethanol in the water had no effect on the TPC recovery but when using 30% methanol, increasing the temperature to 60 °C resulted in a 20% increase in the TPC. This was attributed to methanol being better able to solubilize more polar compounds. The use of microwave-assisted extraction was compared with the solvent extractions using conventional heating and mechanical shaking with the optimized solvent mixtures. The microwave procedure increased the ORAC activity to 2789 μmol Trolox equivalents per gram when using the 30% ethanol in water mixture as the extraction solvent. The main savings in this procedure is the time involved was less without compromising the activity of the extracts. Optimization of extraction using responsive surface methodology was also used to find the most effective conditions to extend oil shelf life [48]. Using ethanol in water over a range of 20% to 100% with a variation of time from 5 to 150 min and temperature from 25° to 90 °C, the study used both the DPPH and the 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS+) assays to determine the optimal conditions. It was found that length of time was not of significance but that temperatures above 60 °C with 75% ethanol in water were optimum. In addition, soybean oil with the optimized extract added at 25 to 1000 mg/kg was subjected to accelerated conditions of agitation at 60 °C and compared to the addition of 200 mg/kg of butylated hydroxytoluene (BHT), a synthetic antioxidant compound. It required 750 mg/kg of the optimal extract to produce the same antioxidant effect of 200 mg/kg of BHT, the synthetic chemical antioxidant.
Isolation of specific proanthocyanidins was performed using preparative HPLC [49]. This allowed for more automated separation of peanut skin extracts. Using earlier work as a guide [21][32], acetone in water (60%) that had been acidified was used as the extractant. An initial purification was performed using Amberlite resin eluted with methanol. This was followed by fractionation on Sephadex LH-20. The fractions were evaluated using the DPPH assay and the most active fraction was further purified using preparative HPLC. This allowed for the isolation of proanthocyanidins A1 and A2 with the major portion of the isolate being the A1 form. The most recent study of the composition of peanut skin extracts compared them to grape skin extracts [50]. The skins were extracted using 80% ethanol in water following a procedure previously published [44]. The extracts were purified using AB-8 resin to purify the procyanidins. The extracts were then evaluated using ORAC, DPPH, ABTS+, and the Ferric Reducing Antioxidant Power (FRAP) assays as well as a cell assay using Human hepatocellular carcinoma (HepG2 cells). Both the A- and the B-type of the procyanidins were identified by HPLC-MS with the A-type predominating as found by others. In most of the assays, the peanut skin extracts had slightly higher values than the grape skin extracts, with the exception of the cell-based assay where the grape skin extracts scored higher. To release the phenolics from peanut skins by the degradation of the actual cell walls for the skins, enzymatic digestion was used [51]. Cellulase was used at an incubation temperature of 55 °C followed by extraction with aqueous ethanol at 45%. The yield of proanthocyanidins was 16.17%.
An exploration of the effectiveness of heat in increasing the solubility and thus the extraction efficiency of solvent in removing phenolic compounds from peanut skins was performed. As many of these compounds are found to be bound into sugars and other cellular structures, the authors theorized that heat treatment of the skins before extraction would increase the yield [52]. Using temperatures from 90 °C to 180 °C, peanut skins from the market types, runner, Virginia, and Spanish were extracted using 70% ethanol in water solutions. TPC and antioxidant activity using the Trolox equivalent antioxidant capacity (TEAC) and the Peroxy-radical trapping capacity assays were compared. Heating to 135 °C resulted in the highest TPC in the runner peanuts (280 mg/g). Both the Virginia and Spanish peanuts did not show any significant change in TPC with heating within the tested range and were both lower (149 mg/g for the Virginia type and 137 mg/g for the Spanish type) than the runner type. Correspondingly, the activity of the peanut skin extracts was not significantly different between the market types (1.38 mg TEAC/g dry skin), except for the higher value of the runner type peanuts heated at 135 °C (2.56 mg TEAC/g dry skin). Only the runner type peanut skins showed any significant Peroxy-radical trapping capacity. The increase in TPC in the runner type was attributed to the formation of Maillard products due to the heat treatment and/or the liberation of phenolics from larger complexes. The differences in market types were attributed to different types of phenolic compounds or complexes being present and some may have been more labile to heat.
2.3. “Green” Extractions
Supercritical extraction uses pressure to condense a solvent or solvent mixture with temperatures above the boiling point of the solvents so that the dielectric constant is decreased [53]. This results in a change in the polarity so that it becomes similar to solvents such as methanol, ethanol, and acetone at room temperature. This allows for the use of more “green” or less toxic chemicals to be used with enhanced solubility of phenolic compounds in the case of peanut skins. A study using this technology optimized the total phenolic content of extracts from peanut skins to be 10 min at 220 °C using 60.5% ethanol in water [53]. This shows the potential of using a more environmentally friendly technique. Further study with this technique to extract peanut skins determined the effect of the size of the particles of peanut skin extracted [54]. Peanut skins milled to particle sizes of 300, 355, 425, and 500 μM were extracted using both traditional Soxhlet extraction and supercritical extraction using carbon dioxide. Optimum yields per weight were obtained by both methods when the particle size was 425 μM and pure ethanol was the solvent compared to either pure water or n-hexane. Although the yield from the Soxhlet extraction using ethanol was higher (36.38% by weight) than carbon dioxide using the supercritical system (15.53%), the antioxidant activity using the DPPH assay was higher for the supercritical system (93.43%) than for the Soxhlet extraction (62.11%). This was attributed to less temperature degradation of the polyphenols by the lower temperatures of operation for the supercritical system. The catechin recovery from this system was higher (139.92 μg/g peanut skin) compared to Soxhlet extraction (31.79 μg/g peanut skin) when ethanol was used as the solvent [55]. More recently, this system has been used to prepare extracts for investigations into the antioxidant protection in cell systems [56]. The extracts were compared to the activity of quercetin, which was thought to be the main compound present. After proving the additives were not cytotoxic up to 250 μg/mL, O2− scavenging activity was assayed. Quercetin was assayed to be seven times higher than the peanut skin extract, which was attributed to the pro-oxidant properties of the catechins present in those extracts. The addition of the quercetin and the peanut skins along with an oxidative stressor was proven to be protective against oxidative stress, whereas pretreatment was not.
A system that used ultrasound was applied to peanut skins with the specific goal of recovering the stilbenoid, resveratrol was reported [57]. Yeast CICC1912, Aspergillus oryzae 3.951, and Aspergillus niger 3.3148 were immobilized onto cellulose beads. Peanut skins were extracted with 80% ethanol in water and the extract was dried. Optimization of the recovery of resveratrol was performed using several surfactants, liquid-solid ratios, ultrasonic powers, and culture temperatures and times. At the optimized conditions, recovery of 96.58 μg/g of resveratrol was obtained, which was four-fold higher than from an untreated sample.
This entry is adapted from the peer-reviewed paper 10.3390/app10238546
References
- United States Department of Agriculture (USDA). International Product Assessment Division. Available online: https://ipad.fas.usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=2221000&sel_year=2020 (accessed on 20 October 2020).
- Zhao, X.; Chen, J.; Du, F. Potential use of peanut by-products in food processing: A review. J. Food Sci. Technol. 2012, 49, 521–529.
- Hale, O.M.; McCormick, W.C. Value of peanut skin (testa) as a feed ingredient for growing-finishing swine. J. Anim. Sci. 1981, 53, 1006–1010.
- Hill, G.M.; Utley, P.R.; Newton, G.L. Influence of dietary crude protein on peanut skin digestibility and utilization by feedlot steers. J. Anim. Sci. 1986, 62, 887–894.
- Hill, G.M.; Utley, P.R.; Newton, G.L. Digestibility and utilization of ammonia-treated and urea-supplemented peanut skin diets fed to cattle. J. Anim. Sci. 1986, 63, 705–714.
- Taş, A.G.; Gökmen, V. Phenolic compounds in natural and roasted nuts and their skins: A brief review. Curr. Opin. Food Sci. 2017, 14, 103–109.
- Bodoira, R.; Maestri, D. Phenolic compounds from nuts: Extraction, chemical and profiles and bioactivity. J. Agric. Food Chem. 2020, 68, 927–948.
- Stansbury, M.F.; Field, E.T.; Guthrie, J.D. The tannin and related pigments in the red skins (Testa) of peanut kernels. J. Amer. Oil Chem. Soc. 1950, 27, 317–321.
- Robinson, G.M.; Robinson, R. XXXI. A survey of anthocyanins. III. Notes on the distribution of leuco-anthocyanins. Biochem. J. 1933, 27, 206–212.
- Randall, J.M.; Hautala, E.; McDonald, G. Binding of heavy metal ions by formaldehyde-polymerized peanut skins. J. Appl. Poly. Sci. 1978, 22, 379–387.
- Paulsen, M.R.; Brusewitz, G.H.; Clary, B.L.; Odell, G.V.; Pominski, J. Aflatoxin content and skin removal of Spanish peanuts as affected by treatments with chemicals, water spray, heated air, and liquid nitrogen. J. Food Sci. 1976, 41, 667–671.
- Sanders, T.H. Changes in tannin-like compounds of peanut fruit parts during maturation. Peanut Sci. 1977, 4, 51–53.
- Karchesy, J.J.; Hemingway, R.W. Condensed tannins: (4β8; 2βO-7)-linked procyanidins in Arachis hypogaea L. J. Agric. Food Chem. 1986, 34, 966–973.
- Pominski, J.; McCourtney, E.J.; Stansbury, M.F.; D’Aquin, E.L.; Vix, H.L.E. Lye-dipping for the removal of objectionable skin color from various grades of shelled Spanish peanuts. J. Amer. Oil Chem. Soc. 1951, 28, 513–516.
- Liu, Z.; Yang, Q.; Zhang, C.; Zhang, Y.; Wang, S.; Sun, J. Study on antioxidant activity of proanthocyanidins from peanut skins. Adv. Mater. Res. 2011, 197, 1582–1586.
- Nepote, V.; Grosso, N.R.; Guzmán, C.A. Antioxidant activity of methanolic extracts from peanut skin. Molecules 2000, 5, 487–488.
- Nepote, V.; Grosso, N.R.; Guzmán, C.A. Extraction of antioxidant components from peanut skins. Grasas Aceites 2002, 53, 391–395.
- Lou, H.; Yamazaki, Y.; Sasaki, T.; Urchida, M.; Tanaka, H.; Oka, S. A-type proanthocyanidins from peanut skins. Phytochemistry 1999, 51, 297–308.
- Whatcott, C.J.; Han, H.; Posner, R.G.; Hostettler, G.; von Hoff, D.D. Targeting the tumor microenvironment in cancer. Why hyaluronidase deserves a second look. Cancer Discov. 2011, 1, 291–296.
- Lou, H.; Yuan, H.; Yamazaki, Y.; Sasaki, T.; Oka, S. Alkaloids and flavonoids from peanut skins. Planta Med. 2001, 67, 345–349.
- Lou, H.; Yuan, H.; Ma, B.; Dongmei, R.; Mei, J.; Oka, S. Polyphenolics from peanut skin and their free radical-scavenging effects. Phytochemistry 2004, 65, 2391–2399.
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds—Nature, occurrence, dietary intake and effects on nutrition on health. J. Sci. Food Agric. 2000, 80, 1094–1117.
- Verstraeten, S.V.; Hammerstone, J.F.; Keen, C.L.; Fraga, C.G.; Oteiza, P.I. Antioxidant and membrane effects of procyanidin dimers and trimers isolated from peanut and cocoa. J. Agric. Food Chem. 2005, 53, 5041–5048.
- Dudek, M.K.; Gliński, V.B.; Davey, M.H.; Silva, D.; Kaźmierksi, S.; Gliński, J.A. Trimeric and tetrameric A-type procyanidins from peanut skins. J. Nat. Prod. 2017, 80, 415–420.
- Chukwumah, Y.; Walker, L.T.; Verghese, M. Peanut skin color: A biomarker for total polyphenolic content and antioxidative capacities of peanut cultivars. Int. J. Mol. Sci. 2009, 10, 4941–4952.
- Shem-Tov, Y.; Bedani, H.; Segev, A.; Hedvat, I.; Galili, S.; Hovav, R. Determination of total polyphenol, flavonoid, and anthocyanin contents and antioxidant capacities of skins from peanut (Arachis hypogaea) lines with different skin color. J. Food Biochem. 2012, 36, 301–308.
- Kuang, Q.; Yu, Y.; Attree, R.; Xu, B. A comparative study on anthocyanin, saponin, and oil profiles of black and red seed coat peanut (Arachis hypogacea (sic)) grown in China. Int. J. Food Prop. 2017, 20, 131–140.
- Attree, R.; Du, B.; Xu, B. Distribution of phenolic compounds in seed coat and cotyledon, and their contribution of antioxidant capacities of red and black seed coat peanuts (Arachis hypogaea L.) Ind. Crops Prod. 2015, 67, 448–456.
- Zhao, Z.; Wu, M.; Zhan, Y.; Zhan, K.; Chang, X.; Yang, H.; Li, Z. Characterization and purification of anthocyanins from black peanut (Arachis hypogaea L.) skin by combined column chromatography. J. Chromatogr. A 2017, 1519, 74–82.
- Huang, J.; Xing, M.; Li, Y.; Cheng, F.; Gu, H.; Yue, C.; Zhang, Y. Comparative transcriptome analysis of the skin-specific accumulation of anthocyanins in black peanut (Arachis hypogaea L.). J. Agric. Food Chem. 2019, 67, 1312–1324.
- Francisco, M.L.L.D.; Resurreccion, A.V.A. Development of a reversed phase high performance liquid chromatography (RP-HPLC) procedure for the simultaneous determination of phenolic compounds in peanut skin extracts. Food Chem. 2009, 117, 356–363.
- Sarnoski, P.J.; Johnson, J.V.; Reed, K.A.; Tanko, J.M.; O’Keefe, S.F. Separation and characterisation of proanthocyanidins in Virginia type peanut skins. Food Chem. 2012, 131, 927–939.
- Appeldoorn, M.M.; Vincken, J.-P.; Sanders, M.; Hollman, P.C.H.; Gruppen, H. Combined normal-phase and reversed-phase liquid chromatography ESI-MS as a tool to determine the molecular diversity of A-type procyanidins in peanut skins. J. Agric. Food Chem. 2009, 57, 6007–6013.
- Xu. Q.; Ju, Y.; Ge, H, Research on resin separation technology of resveratrol and catechin from peanut skin. Adv. Mater. Res. 2012, 610–613, 3450–3460.
- Zhang, H.; Yang, Y.Y.; Ma, C. Structures and antioxidant and intestinal disaccharidase inhibitory activities of A-type proanthocyanidins from peanut skin. J. Agric. Food Chem. 2013, 61, 8814–8820.
- Longo, E.; Rossetti, F.; Merkyte, V.; Bosselli, E. Disambiguation of isomeric procyanidins with cyclic B-type and non-cyclic A-type structures from wine and peanut skins with HPLC-HDX-HRMS/MS. J. Am. Soc. Mass Spectrom. 2018, 29, 2268–2277.
- Chukwumah, Y.; Walker, L.; Volger, A.; Verghese, M. Changes in the phytochemical composition of raw, boiled, and roasted peanuts. J. Agric. Food Chem. 2007, 55, 9266–9273.
- Chukwumah, Y.; Walker, L.; Volger, A.; Verghese, M. Profiling of bioactive compounds in cultivars of Runner and Valencia peanut market-types using liquid chromatography/APCI mass spectrometry. Food Chem. 2012, 132, 525–531.
- Ma, Y.; Kosińska-Cagnazzo, A.; Kerr, W.L.; Amarowicz, R.; Swanson, R.B.; Pegg, R.B. Separation and characterization of phenolic compounds from dry-blanched peanut skins by liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. A 2014, 1356, 64–81.
- Yu, J.; Ahmedna, M.; Goktepe, I. Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chem. 2005, 90, 199–206.
- Ma, Y.; Kosińska-Cagnazzo, A.; Kerr, W.L.; Amarowicz, R.; Swanson, R.B.; Pegg, R.B. Separation and characterization of soluble esterified and glycoside-bound phenolic compounds in dry-blanched peanut skins by liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2014, 62, 11488–11504.
- Ballard, T.S.; Mallikarjunan, P.; Zho, K.; O’Keefe, S.F. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010, 120, 1185–1192.
- Ballard, T.S.; Mallikarjunan, P.; Zho, K.; O’Keefe, S.F. Optimizing the extraction of phenolic antioxidant compounds from peanut skins using response surface methodology. J. Agric. Food Chem. 2009, 57, 3064–3072.
- Nepote, V.; Grosso, N.R.; Guzmán, C.A. Optimization of phenol antioxidants from peanut skins. J. Sci. Food Agric. 2005, 85, 33–38.
- Liu, Z.Q.; Yang, Q.L.; Zhang, C.S.; Sun, J.; Zhang, Y.; Wang, S.Q. Optimization of ultrasonic extraction technique of proanthocyanidin from peanut skin. Adv. Mater. Res. 2010, 156–157, 778–784.
- Takano, F.; Takata, T.; Yoshihara, A.; Nakamura, Y.; Ohta, T. Aqueous extract of peanut skin and its main constituent procyanidin A1 suppress serum IgE and IgG1 levels in mice-immunized with ovalbumin. Biol. Pharm. Bull. 2007, 30, 922–927.
- Ballard, T.S. Optimizing the Extraction of Phenolic Antioxidant Compounds from Peanut Skins. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2008.
- Franco, D.; Rodríguez-Amado, I.; Agredán, R.; Mundkata, P.E.S.; Vázquez, J.A.; Barba, F.J.; Lorenzo, J.M. Optimization of antioxidants extraction from peanut skin to prevent oxidative processes during soybean oil storage. LWT Food Sci. Technol. 2018, 88, 1–8.
- Oldoni, T.L.C.; Melo, P.S.; Massarioli, A.P.; Moreno, I.A.M.; Bezerra, R.M.N.; Rosalen, P.L.; da Silva, G.V.J.; Nascimento, A.M.; Alencar, S.M. Bioassay-guided isolation of proanthocyanidins with antioxidant activity from peanut (Arachis hypogaea) skin by combination of chromatography techniques. Food Chem. 2016, 192, 306–312.
- Chang, M.; Sun, X.; Bai, H.; Liu, R.; Jin, Q.; Wang, X. Composition and antioxidant study of procyanidins from peanut skins. J. Food Meas. Charact. 2020, 14, 2781–2789.
- Zang, C.S.; Yang, Q.L.; Jia, K.; Liu, Z.Q.; Yu, L.N.; Sun, J. Research on extraction of proanthocyanidin from peanut skin by Aspergillus niger fermentation. Adv. Mater. Res. 2011, 236–238, 2499–2504.
- Francisco, M.L.L.D.; Resurreccion, A.V.A. Total phenolics and antioxidant capacity of heat-treated peanut skins. J. Food Comp. Anal. 2009, 22, 16–24.
- Bodoira, R.; Rossi, Y.; Monenegro, M.; Maestri, D.; Velez, A. Extraction of antioxidant polyphenolic compounds from peanut skin using waste-ethanol at high pressure and temperature conditions. J. Supercrit. Fluids 2017, 128, 57–65.
- Putra, N.R.; Rizkiyah, D.N.; Zaini, A.S.; Yunus, M.A.C.; Machmudah, S.; Idham, Z.b.; Ruslan, M.S.H. Effect of particle size on yield extract and antioxidant activity of peanut skin using modified supercritical carbon dioxide. J. Food Process. Preserv. 2018, 42, e13689.
- Putra, N.R.; Yunus, M.A.C.; Ruslan, M.S.H.; Idham, Z.; Idrus, F.N. Comparison extraction of peanut skin between CO2 supercritical fluid extraction and Soxhlet extraction in terms of oil yield and catechin. Pertankia, J. Sci. Technol. 2018, 26, 799–810.
- Rossi, Y.E.; Bohl, L.P.; Vanden Braber, N.L.; Ballatore, M.B.; Escobar, F.M.; Bodoira, R.; Maestri, D.M.; Porporatto, C.; Cavaglieri, L.R.; Montenegro, M.A. Polyphenols of peanut (Arachis hypogaea L.) skin as bioprotectors of normal cells. Studies of cytotoxicity, cytoprotection and interactions with ROS. J. Funct. Foods 2020, 67, 103862.
- Jin, S.; Gao, M.; Kong, W.; Yang, B.; Kuang, H.; Yang, B.; Fu, Y.; Cheng, Y.; Li, H. Enhanced and sustainable pretreatment for bioconversion and extraction of resveratrol from peanut skin using ultrasound-assisted surfactant aqueous system with microbial consortia immobilized on cellulose. 3 Biotech 2020, 10, 293.
