The hippocampus is crucial in learning, memory and emotion processing, and is involved in the development of different neurological and neuropsychological disorders. Several epigenetic factors, including DNA methylation, histone modifications and non-coding RNAs, have been shown to regulate the development and function of the hippocampus, and the alteration of epigenetic regulation may play important roles in the development of neurocognitive and neurodegenerative diseases.
- epigenetics
- hippocampus
- radiation
- histones
- neurogenesis
- miRNA
- DNA methylation
1. Introduction
The hippocampus is a structure in the brain within the medial temporal lobe [1] which plays an important role in the limbic system and is involved in learning, memory processing and emotions [2]. The three principle structures of the hippocampal formation (hippocampus, dentate gyrus and subiculum) are made up of glutamatergic principal neurons and inhibitory GABAergic neurons [3], and damage to the hippocampus has been shown to bring about amnesic effects in humans, alluding to its major role in memory formation and consolidation [4]. Both animal and clinical studies have clearly indicated that radiation exposure or radiotherapy induces hippocampal damage, resulting in the impairment of neurogenesis and cognition [5]. The production of reactive oxygen species (ROS), pro-inflammatory cytokines and chemokines, pro-apoptotic proteins, autophagosome markers, excitatory neurotransmitters and neurotrophic factors may be involved in hippocampal neuropathy and cognitive impairment [6,7]. Recent studies have suggested that epigenetic mechanisms are involved not only in normal hippocampal development and regulation [8,9,10,11,12,13,14], but also in radiation-induced hippocampal pathophysiological changes leading to the development of neurological and neuropsychological disorders [15].
2. Epigenetic Regulation of Hippocampal Neurogenesis
The generation of new neurons in the adult central nervous system (CNS) in the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus is one of the major breakthroughs in neuroscience research. Adult hippocampal neurogenesis occurs in the DG and refers to the formation of new functional dentate granule cells from neural stem cells (NSCs) [10] contributing to learning and memory [11] and also mood regulation [54]. Adult hippocampal neurogenesis was first observed in rodents [55] and later confirmed in humans [56,57,58]. This process occurs when intermediate neural progenitors (IPCs) are amplified and integrated into existing neural circuits. Due to this, adult hippocampal neurogenesis provides a means for both functional and structural plasticity in the hippocampus. Dysregulation of adult hippocampal neurogenesis has been shown to cause cognitive decline and psychological symptoms [10].
The process of adult hippocampal neurogenesis is regulated by factors that are both extrinsic and intrinsic [59], which are able to actively upregulate or downregulate the generation of new neurons throughout adulthood and may occur prenatally or postnatally. One of the key regulators of neurogenesis is epigenetics. Studies have shown that epigenetic regulators are crucial for the generation of neurons from adult neural progenitors that integrate into the hippocampus [8,26,60,61]. These neural progenitor cells have high levels of histone H3 lysine 4 trimethylation (H3K4me3) and histone H3 lysine 27 trimethylation (H3K27me3); alterations of H3K4me3 and H3K27me3 are often the focus of environmental epigenetic studies due to their strong association with gene expression at promoters. Histone deacetylase 1 (HDAC1) is mainly expressed in NSCs, while mature neurons mainly express histone deacetylase 2 (HDAC2) [62] suggesting that the expression of HDACs may be developmentally regulated. Combined deletion of both these HDACs resulted in an inability for neuronal precursors to differentiate into mature neurons, leading to excessive cell death [63]. In cases where HDAC2 was depleted, studies have shown that HDAC1 was able to compensate for the loss of HDAC2 [64], and thus, neurogenesis is not affected. In terms of histone acetylation, KAT6B, a gene which provides instructions for making histone acetyltransferases, is highly expressed in the adult SVZ, and a deficiency of KAT6B results in a decrease in NSCs [65].
Tritorax group (trxG) and polycomb group (PcG) proteins activate or silence gene expression respectively, through a chromatin remodeling system [66]. Epigenetic modifications of chromatin structure by PcG proteins, which function as transcriptional repressors, aid in the maintenance of cellular identity [67]. These proteins facilitate the trimethylation of the lysine 27 of histone 3 (H3K27me3), bringing about transcriptional repression, and trxG protein complexes catalyze the trimethylation of H3K4 (H3K4me3) [11]. Mixed-lineage leukemia I (Mll1) is a member of the trxG family and it is expressed in the SVZ and olfactory bulb (OB) [68]. Mll1 has been shown to be crucial for the proliferation and neurogenesis of SVZ and the olfactory bulb (OB) NSCs [66] and is known to be a histone methyltransferase (HMT) for histone H3 lysine 4 [69]. Studies have shown that a deficiency of Mll1 in the SVZ severely impaired neuronal differentiation [66].
Sex determining region Y-box 2 (SOX2) has been reported to prime the epigenetic landscape in neural progenitors, as the early SOX2-dependent imbalance in H3K4me3 and H3K27me3 marks has a profound impact throughout the entire differentiation process, which allows for proper gene activation during neurogenesis [8]. The epigenetic mechanisms that regulate neurogenesis are extremely strongly associated with each other and other regulatory pathways [70].
The involvement of DNA methylation is seen in studies with Gadd45b, which is a crucial component for the DNA methylation of certain promoters and their corresponding gene expressions essential for neurogenesis [60], thereby increasing the expression of key neuronal genes such as Fgf1 and Bdnf [65]. Mice with Gadd45b deletions showed deficits in the proliferation of neural progenitors and dendritic growth in the hippocampus [60]. In addition, mice exposed to prenatal stress showed increased expressions of DNA methyltransferase 1 (DNMT1), and also an increase in its binding to the glutamic acid decarboxylase 67 (GAD67) promoter, leading to an impairment in the genesis of the gamma-aminobutyric acid (GABA) interneurons, suggesting that prenatal upregulation of DNMT1 may reduce interneuron genesis [10]. On the other hand, the expression of DMNT1 in neural precursor cells was also shown to be crucial for the survival of newly generated neurons in the adult hippocampus. Deletion of DNMT1 in NSCs at an early stage of DG development impaired the ability of NSCs to establish secondary radial glial scaffolds and to migrate into the SGZ of the DG, leading to aberrant neuronal production in the molecular layer, increased cell death and decreased granule neuron production. Furthermore, it promoted the differentiation of NSCs into astrocytes [71,72]. In addition to DMNT1, DMNT3-knockout mice were also found to have significantly fewer immature neurons [73]. DNMT1 has been shown to control the timing of astrogliogenesis through Janus kinase/signal transducers and activators of transcription (JAK-STAT) signaling, and DNMT3A and DNMT3B are required for neuron specification [65]. Maternal exposure to 3,3’-iminodipropionitrile (IDPN) was shown to affect hippocampal neurogenesis in offspring, and this was found to be due to hypermethylation of genes Edc4, Kiss1 and Mrpl38 [13]. DMNTs have been proven to be crucial in ensuring normal neurogenesis, as dysregulation or mutations in DNMTs result in abnormal neurogenesis [74] which further confirms the importance of DNA methylation in hippocampal neurogenesis. Reductions in hippocampal neurogenesis have also been linked to glucocorticoid hormones (GC) which regulate neural stem/precursor cell proliferation via changes in the methylation state of gene promoters associated with cell cycle regulation and Wnt signaling [75]. Disruption of GCs causes alterations in dendritic morphology and numbers and the appearance of new granule neurons.
Methyl-CpG-binding protein 2 (MeCP2) is a member of the methyl-CpG binding domain (MBD) family that plays an important role in both neuronal and astrocytic lineage specification by repressing astrocytic genes during neurogenesis and releasing this repression during astrogenesis [76]. MeCP2 appears to play a key role in conveying neuronal signaling and activity into epigenetic gene regulation. MeCP2-knockout mice have neurons with smaller nuclei and fewer dendritic branches [77], and activity-dependent changes in DNA methylation were associated with a reduction in MeCP2 binding [78]. MeCp2 deficiency also correlated with poor neural progenitor cell (NPC) maturation and impaired dendritic and spine morphogenesis in new neurons [79]. In addition to MeCP2, MBD1 proteins of the MBD family have also been shown to play crucial roles in hippocampal neurogenesis [70], and it is their MBD domains that interact with methylated CpG, and thus, MBD binding correlates with DNA methylation. MBD1-deficient mice had lower levels of neurogenesis and impaired spatial learning capabilities [80] and were also found to be susceptible to depression [81]. It is evident that DNA methylation has a crucial role in the maintenance of NPCs and their fate specification in adult hippocampal neurogenesis.
It has also been shown that MeCP2 is able to epigenetically regulate miRNAs in adult NSCs, which brings in another form of epigenetic regulation. Co-regulation of miR-137 together with SOX2 regulates the proliferation and differentiation of adult neural stem cells [82]. Knockdown of miR-137 enhances their differentiation while overexpression of miR-137 promotes their proliferation. Furthermore, miR-137 has been found to repress the expression of enhancer of zeste homolog 2 (EZH2), a PcG histone methytransferase, which brings about a global reduction in H3K27me3-modulated neurogenesis [82]. Similarly, high levels of miR-184 were found to promote proliferation of neural progenitors while inhibiting their differentiation [83].
The miR-30 family of miRNAs has also been shown to mediate the effects of stress on hippocampal neurogenesis in mice [84]. These miRNAs were found to be downregulated in stressed mice and involved in neural progenitor cells differentiation. In addition, miR-137 and miR-34a have been shown to negatively regulate dendritic branching and the complexity of newborn neurons [85,86], and miR-19 has been proven to be crucial for the migration of these newborn neurons [87]. Human neural progenitor cells with abnormal expression of miR-19 display deviant migration patterns. Regulation of ten-eleven translocation protein 1 (TET1), a methylcytosine dioxygenase, and miR-124 has also been shown to modulate hippocampal neurogenesis. Together with miR-9, miR-124 appears to repress Brg- and Brahma (Brm)- associated factor-complex 53a (BAF53a) allowing neural progenitors to properly differentiate into neurons [69]. Thus, miR-124 is very lowly expressed in progenitor cells but upregulated in differentiation and mature neurons [88]. TET1 controls the demethylation of miR-124 thereby regulating its expression. Dysregulation of TET1 and miR-124 due to Down Syndrome critical region 1 (DSCR1) protein knockout led to an impairment in hippocampal neurogenesis [89]. TET1 is also known to interact with MeCP2 further confirming the importance of epigenetic regulation including the crosstalk between different epigenetic factors [90]. Several studies also show that a synergistic modulation by various different miRNAs facilitates hippocampal neurogenesis and is crucial for neurogenic lineage fate determination in the adult hippocampus [91,92,93]. Epigenetic factors governing adult hippocampal neurogenesis are summarized in Figure 1. We are just beginning to understand the influence of epigenetics on hippocampal neurogenesis as an intermediate regulatory mechanism between DNA sequences and gene expression. Thus, further studies should be carried out on the molecular pathways to induce, remove and interpret epigenetic modifications. Looking downstream, this may enable us to further elucidate the mechanisms of neurodevelopment-related disorders and aid the development of novel therapeutic approaches to prevent abnormal brain development.
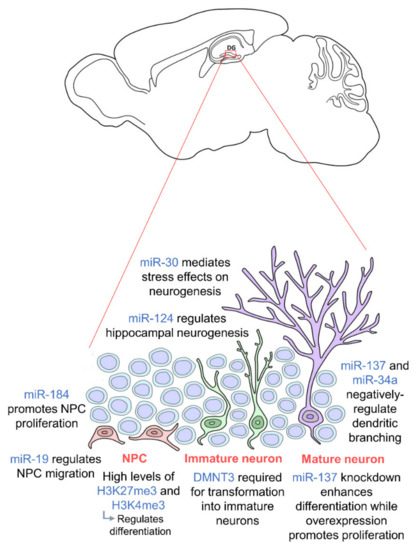
Figure 1. Epigenetic regulation during adult hippocampal neurogenesis. Adult hippocampal neurogenesis is regulated by epigenetics at every stage. Neural progenitor cells (NPCs) (orange) require high levels of H3K27me3 and H3K4me3 for proper differentiation, and their proliferation and migration are regulated by miR-184 and miR-19 respectively. In order for NPCs to transform into immature neurons (green), DMNT3 is required. Mature neurons (purple) are then regulated by miR-137 and miR-34a. miR-30 and miR-124 regulate adult hippocampal neurogenesis in general. NPCs are located in the subgranular zone (SGZ) of the dentate gyrus, and mature neurons integrate into the granule cell layer. (DNMT: DNA methyltransferase).
This entry is adapted from the peer-reviewed paper 10.3390/ijms21249514
