Probiotics and synbiotics are known to have beneficial effects on human health and disease. Hirsutism, a disorder that is characterised by the presence of coarse terminal hairs in a male-like pattern, is usually caused by elevated androgen levels in blood plasma. This disorder is usually ob-served in PCOS women and it is linked to insulin resistance (IR). Although idiopathic hirsutism (IH) is not shown to have excess androgen production from the ovarian and adrenal glands, in-creased 5α-reductase in peripheral tissues and insulin resistance are common observations.
- probiotics
- synbiotics
- hirsutism
- androgens
- insulin resistance
- PCOS
1. Introduction
Fermented food, such as yoghurt, bread, bear and wine, has been widely known since ancient times, and some have been used since then, for therapeutic purposes [1][2][3]. Fermented dairy products (yoghurt, kefir) were used to treat diseases like diarrhea and other infections of the intestinal tract [4][5]. Interaction between microorganisms and human health was first reported in 1907, where yoghurt microflora was described by Elie Metchnikoff [6]. Fermented food is still used as an additional treatment for disease, such as wounds [7][8][9], gastroenteric disorders and infections [10], as well as an overall support for health [10][11][12]. The term probiotic was introduced by Werner Kollath [13] and according to the World Health Organisation (WHO), this term refers to the microorganisms that ‘’when administered in adequate amounts, confer a health benefit on the host’’ [14]. Probiotics are found to have multiple beneficial roles on human health and disease, through restoration of gut microbiota (GM) [15][16], symptom improvement on intestinal disease, such as irritable bowel syndrome (IBS) [17], modulation of hormonal profile in animals and humans [18][19][20][21][22] and skin infections and healing [23][24][25]. Along with the beneficial microorganisms, prebiotics, which are fermented compounds that support and promote modifications in the activity and composition of GM [26], have also been studied. It is shown that a controlled combination of probiotics and prebiotics, called synbiotics, can provide a more enhanced beneficial effect on human health and disease [27][28][29].
Although the effect of probiotics and synbiotics are extensively studied, there has been limited research conducted on their effect on sex hormones and sex hormone imbalances, such as hirsutism, a disorder that is mainly characterised by elevated androgen levels in women. The aim of this review is to focus on the role of probiotics and synbiotics and their metabolic process on hirsutism.
2. Probiotics
Lactobacillus, Streptococcus, Bifidobacterium, Lactococci and Saccharomyces are some of the probiotic species that are known [30]. Microorganisms that are mostly used as treatments are Lactobacillus, Streptococcus, Bifidobacterium and Saccharomyces, whilst most of them are naturally present in the human GM [31][32][33][34][35][36][37]. There has been an increase in research on the effect of probiotics on health and disease, whilst they are shown to have both beneficial effects on healthy subjects and therapeutic effect on various diseases and conditions, such as intestinal disease [38][39][40][41][42][43][44][45][46], skin disease [47][48][49][50][51][52] and wound healing [53][54][55][56][57]. Probiotics have also been used for the restoration of disturbed GM [58]. Dysbiosis of gut microbiota (DOGMA) is the alteration of gut microflora that can occur due to a variety of reasons, such as antibiotic use [59][60] and diet [61][62]. The human gut, also described as the ‘’second brain’’ of the human body, plays an important role in human health and disease [63][64]. The GM is known to have a significant role in the functionality of the bowel, maintaining the gut mucosa through their role on gut homeostasis [63][64][65]. The probiotic E. coli Nissle 1917 protects and prevents against inflammatory response via TLR-4 and TLR-2-dependent pathways, whilst lower counts of Firmicutes prausnitzii are linked with potential inflammatory bowel disease pathogenesis due to its anti-inflammatory effects [65][66]. The impact of the metabolic processing of the gut microflora in the gut is extended outside of the bowel and can impact other functionalities of the human body, reaching the skin [67][68][69]. L. casei is shown to reduce skin inflammation through the regulation of CD8+T cells, which initiate inflammatory response. The role of probiotics on endocrinology has also been reported and GM was shown to affect the production of hormones, such as leptin [70][71], stress hormones [72], insulin [73][74] and sex hormones [75][76] in the intestinal tract. Additionally, the disturbance of gut microbiota can affect the levels of endogenous hormones, such as estrogens, as well as administrated steroids, such as megestrol acetate, medroxyprogesterone acetate, norethisterone and others, suggesting that the use of antibiotics can cause hormonal imbalance and reduce the absorption of contraceptive hormones, which are used as a method of birth control and/or treatment for metabolic syndromes [75]. An altered microbiota during the early life of the diabetic mouse (Type 1) can determine sex hormones and cause metabolic changes, such as increased testosterone levels [76]. Administration of probiotics can restore the imbalanced microorganisms that live in the human intestine and improve certain conditions through their metabolic processes. Additionally, DOGMA is also observed in patients suffering from inflammatory bowel disease, colitis [77], Crohn’s disease, IBS, polycystic ovary syndrome (PCOS) [78] and other conditions.
3. Synbiotics
Synbiotics are the controlled combination of probiotics and prebiotics and their supplementation aims to provide a more enhanced health benefit on human health and disease. Known prebiotics are fructans (e.g. inulin), complex polysaccharides, oligosaccharides and sugar alcohols [79] and in combination with probiotics, they have been used for the treatment of conditions and disease [80][81]. Synbiotics containing L. acidophilus NCC90, oligofructose and acacia gum can have a preventive role on bone mineral loss after ovariectomy in rats. It has been reported that synbiotic treatments showed beneficial effects on non-alcoholic fatty liver disease (NAFLD), reducing fibrosis and hepatic steatosis in humans [82][83], total necrosis factor α (TNF-α), total nuclear factor κ-B (TNF-κB) and other NAFLD biomarkers, such as high-sensitivity C-reactive protein [83]. Moreover, synbiotics can bring improvements on Crohn’s disease through reduction in TNF-α production [84], IBS [85] and delayed Alzheimer’s disease in Drosofila melanogaster [86]; improvement of thyroid function was observed after 8 weeks of synbiotic supplementation in hypothyroid patients, decreasing thyroid stimulating hormone (TSH) levels and increasing tri-iodothyronine (FT3), whilst overall increasing FT3/TSH ratio. Bifidobacterium Longum in combination with inulin, reduced levels of TNF-α and IL-1α in patients suffering from ulcerative colitis. Another study on patients with ulcerative colitis showed that B. breve and galacto-oligosaccharide (GOS)-containing beverage (Yakult) managed to clinically improve the condition of the patients, through reduction of UC markers, such as myeloperoxidase, and by lowering the faecal pH [87].
4. Hirsutism
Hirsutism is a condition that appears in 5–10% of women and it is recognized by the presence of coarse terminal hairs in a male-like pattern [88]. Excess hair growth that consists of terminal hairs are present in areas were women normally have thinner hair. The clinical diagnosis of hirsutism is completed based on Ferriman and Gallwey criteria
(Figure 1) [88] and the areas that are scored include the face, chest, thighs, upper arms, abdomen and back. Scores (m-FG) from 1–4, with 1 describing minimal terminal hair and 4 describing frank virilization, are given to the mentioned areas whist total scores less than 8 are considered normal [88].
Figure 1. Hirsutism scoring system presenting scores ranking from 1—minimal hirsutism, to 4 virilization in 9 body parts. A total score of less than 8 is considered normal, whereas higher scores indicate mild to severe hirsutism [88].
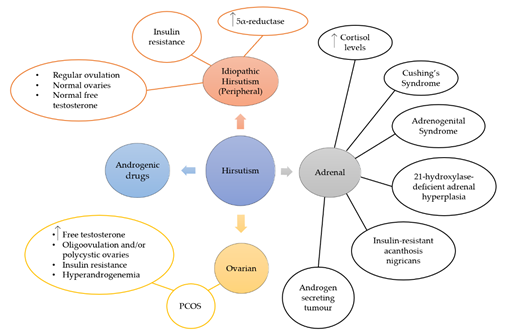
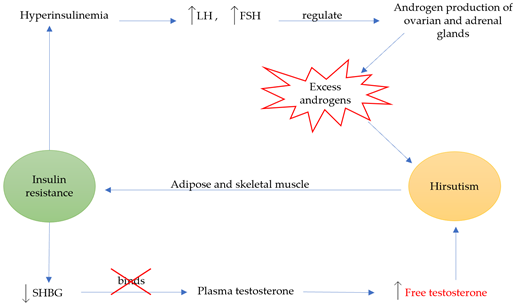
Hirsutism is a quite complex condition that can occur due to other syndromes or disease, such as PCOS (Figure 2). Although the main reason for this condition is the presence of excess androgens, such as testosterone (T) and dihydrotestosterone (DHT), IH shows normal levels of androgens [89][90]. The most common reason for the excess androgen levels is PCOS and 60–80% of PCOS women suffer from hirsutism [91]. The diagnosis of this condition is conducted according to the Rotterdam criteria, by which two of the three criteria have to be met: a) evidence of hyperandrogenism, b) oligo- and/or anovulation and c) polycystic ovaries [92]. Other clinical features are ovarian enlargement and IR, which is found to be very common amongst PCOS women, whilst the syndrome has also been linked with cardiovascular disease and inflammation. The heritability of this disorder has been studied and it is possible that genetic changes on the androgen receptor gene can lead to hirsutism [90]. During this study, a repeatability of the trinucleotide CAG in exon 1 was observed and more frequent repeats in the N-terminal domain of the androgen gene were linked with the development of the disorder. However, other researchers have not shown a significant role of CAG in the pathogenesis of hirsutism [93][94]. Hirsutism has also been linked with IR (Figure 3) [95][96][97][98][99][100][101], whilst excess insulin production leads to hyperinsulinemia, which increases luteinizing hormone (LH) through insulin receptor stimulation [97]. LH, along with follicle-stimulating hormone (FSH), is a hormone that regulates androgen production through the secretion of them from ovarian and adrenal glands. Moreover, high levels of insulin inhibit sex hormone-binding globulin (SHBG), a hormone that binds with plasma T and is considered its major determinant along with 17-β hydroxysteroids from plasma [102]. Consequently, both effects of IR are directly linked with hirsutism through excess androgen production. This relationship between IR and hirsutism was studied in healthy women, where adipose tissue was collected and in-vitro treatments with testosterone and/or anti-androgens were conducted on insulin stimulated adipose cells [100].
Figure 2. Hirsutism can originate from adrenal or ovarian disorders, or both. Various syndromes and disorders related to the adrenal glands, such as Cushing’s syndrome, adrenogenital syndrome, 21-hydroxylase-deficient adrenal hyperplasia, insulin-resistant acanthosis nigricans and androgen secreting tumour, show high levels of cortisol hormone and hirsutism. Ovarian hirsutism is caused by PCOS that is characterised from high levels of free testosterone and/or polycystic ovaries, insulin resistance and hyperandrogenemia. Idiopathic hirsutism is caused by peripheral increase of androgens, and women that suffer from it show increased levels of 5α-reductase and insulin resistance, whilst they do not show any abnormalities in their ovarian or adrenal function.
Adipose tissue contains adipocytes, also known as fat cells and are responsible for fat energy storage. This study showed that the exposure of these cells to testosterone led to insulin resistance, suggesting there is a link between hirsutism, androgen presence and insulin resistance development.
Other disorders that are known for the increased production of androgens are hyperandrogenic insulin-resistant acanthosis nigricans syndrome [103][104], 21-hydroxylase-deficient non-classic adrenal hyperplasia [105], androgen-secreting tumor [106], and rarely androgenic drug intake [107]. On the other hand, the causes of IH are not well known. However, when hormonal profiles of PCOS women and IH women were compared, IR was found to be significant in both groups compared to the control group. IR and hyperinsulinemia increase the insulin-like growth factor (IGF) which affects hair follicles, and therefore it is suggested that IR is potentially a cause of IH. This disorder is also characterised as an increase in 5α-reductase in peripheral tissues, an enzyme that converts T to DHT, and is generally responsible for the metabolism of steroids. Potential causes of IH include increased sensitivity of hair follicles to androgens, and androgen receptor gene polymorphism.
Figure 3. The relationship between insulin resistance and hirsutism. Hyperinsulinemia is caused when insulin resistance is left unmanaged, causing an increase in LH and FSH, which regulate the production of ovarian and adrenal androgens. This increase results in excess androgens, and eventually hirsutism. Moreover, insulin resistance reduces SHBG, which leaves plasma testosterone unbound leading to increased free testosterone and hirsutism. Chronic androgen exposure leads to adipose and skeletal muscle insulin resistance. The graph above presents the circular relationship between the two conditions, leading to a vicious cycle of effects.
This entry is adapted from the peer-reviewed paper 10.3390/fermentation7010010
References
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nunez, A.; Butrym, E.D.; Richards, M.P.; Wang, C. -s.; et al. Fermented beverages of pre- and proto-historic China. Proc. Natl. Acad. Sci. 2004, 101, 17593–17598, doi:10.1073/pnas.0407921102.
- Sicard, D.; Legras, J.L. Bread, beer and wine: Yeast domestication in the Saccharomyces sensu stricto complex. Comptes Rendus - Biol. 2011, 334, 229–236.
- Ozen, M.; Dinleyici, E.C. The history of probiotics: The untold story. Benef. Microbes 2015, 6, 159–165.
- Isolauri, E. Probiotics in human disease. In Proceedings of the American Journal of Clinical Nutrition; 2001; Vol. 73, pp. 1142S-1146S.
- Vandenplas, Y.; Zakharova, I.; Dmitrieva, Y. Oligosaccharides in infant formula: More evidence to validate the role of prebiotics. Br. J. Nutr. 2015, 113, 1339–1344, doi:10.1017/S0007114515000823.
- Gordon, S. Elie Metchnikoff: Father of natural immunity. Eur. J. Immunol. 2008, 38, 3257–3264, doi:10.1002/eji.200838855.
- Rodrigues, K.L.; Gaudino Caputo, L.R.; Tavares Carvalho, J.C.; Evangelista, J.; Schneedorf, J.M. Antimicrobial and healing activity of kefir and kefiran extract. Int. J. Antimicrob. Agents 2005, 25, 404–408, doi:10.1016/j.ijantimicag.2004.09.020.
- Rahimzadeh, G.; Fazeli, M.R.; Mozafari, A.N.; Mesbahi, M. EVALUATION OF ANTI-MICROBIAL ACTIVITY AND WOUND HEALING OF KEFIR | INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES AND RESEARCH Available online: http://ijpsr.com/bft-article/evaluation-of-anti-microbial-activity-and-wound-healing-of-kefir/?view=fulltext (accessed on Nov 26, 2018).
- Atalan, G.; Demirkan, I.; Yaman, H.; Cina, M. Effect of topical kefir application on open wound healing on in vivo study. Kafkas Univ Vet Fak Dderg 2003, 9, 43–47.
- Lolou, V.; Panayiotidis, M.I. Functional role of probiotics and prebiotics on skin health and disease. Fermentation 2019, 5, doi:10.3390/fermentation5020041.
- Sharifi, M.; Moridnia, A.; Mortazavi, D.; Salehi, M.; Bagheri, M.; Sheikhi, A. Kefir: a powerful probiotics with anticancer properties. Med. Oncol. 2017, 34, 183.
- Chen, M.J.; Liu, J.R.; Sheu, J.F.; Lin, C.W.; Chuang, C.L. Study on skin care properties of milk kefir whey. Asian-Australasian J. Anim. Sci. 2006, 19, 905–908, doi:10.5713/ajas.2006.905.
- Collins, M.D.; Phillips Brian A.; Zanoni Paolo Deozyribonucleic acid homology studies of lactobacillus casei etc. Collins 1989. Int. J. Syst. Bacteriol. 1989, 39, 105–108.
- Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; Guidelines for the Evaluation of Probiotics in Food; London ON, Canada, 2002;
- Goldin, B.R.; Swenson, L.; Dwyer, J.; Sexton, M.; Gorbach, S.L. Effect of diet and Lactobacillus acidophilus supplements on human fecal bacterial enzymes. J. Natl. Cancer Inst. 1980, 64, 255–261, doi:10.1093/jnci/64.2.255.
- Scholz-Ahrens, K.E.; Adolphi, B.; Rochat, F.; Barclay, D. V.; de Vrese, M.; Açil, Y.; Schrezenmeir, J. Effects of probiotics, prebiotics, and synbiotics on mineral metabolism in ovariectomized rats - impact of bacterial mass, intestinal absorptive area and reduction of bone turn-over. NFS J. 2016, 3, 41–50, doi:10.1016/j.nfs.2016.03.001.
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, 1–67, doi:10.1128/mmbr.00036-17.
- Talebi, S.; Karimifar, M.; Heidari, Z.; Mohammadi, H.; Askari, G. The effects of synbiotic supplementation on thyroid function and inflammation in hypothyroid patients: A randomized, double‑blind, placebo‑controlled trial. Complement. Ther. Med. 2020, 48, doi:10.1016/j.ctim.2019.102234.
- Nasri, K.; Jamilian, M.; Rahmani, E.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Asemi, Z. The effects of synbiotic supplementation on hormonal status, biomarkers of inflammation and oxidative stress in subjects with polycystic ovaryfile:///C:/Users/Vassoula/Downloads/29664663.nbib syndrome: A randomized, double-blind, placebo-controlled trial. BMC Endocr. Disord. 2018, 18, 1–8, doi:10.1186/s12902-018-0248-0.
- He, M.; Gao, J.; Wu, J.; Zhou, Y.; Fu, H.; Ke, S.; Yang, H.; Chen, C.; Huang, L. Host gender and androgen levels regulate gut bacterial taxa in pigs leading to sex-biased serum metabolite profiles. Front. Microbiol. 2019, 10, 1–13, doi:10.3389/fmicb.2019.01359.
- Yarak, S.; Brasil Parada, M.O.A.; Bagatin, E.; Talarico Filho, S.; Hassun, K.M. Hyperandrogenism and skin: Polycystic ovary syndrome and peripheral insulin resistance. An. Bras. Dermatol. 2005, 80, 395–410.
- Carnevali, O.; Avella, M.A.; Gioacchini, G. Effects of probiotic administration on zebrafish development and reproduction. Gen. Comp. Endocrinol. 2012, 188, 297–302.
- Sonal Sekhar, M.; Unnikrishnan, M.K.; Vijayanarayana, K.; Rodrigues, G.S.; Mukhopadhyay, C. Topical application/formulation of probiotics: Will it be a novel treatment approach for diabetic foot ulcer? Med. Hypotheses 2014, 82, 86–88, doi:10.1016/j.mehy.2013.11.013.
- Sikorska, H.; Smoragiewicz, W. Role of probiotics in the prevention and treatment of meticillin-resistant Staphylococcus aureus infections. Int. J. Antimicrob. Agents 2013, 42, 475–481, doi:10.1016/j.ijantimicag.2013.08.003.
- Eggers, S.; Barker, A.; Valentine, S.; Hess, T.; Duster, M.; Safdar, N. Impact of Probiotics for Reducing Infections in Veterans (IMPROVE): Study protocol for a double-blind, randomized controlled trial to reduce carriage of Staphylococcus aureus. Contemp. Clin. Trials 2017, 52, 39–45, doi:10.1016/j.cct.2016.11.004.
- Frei, R.; Akdis, M.; O’mahony, L. Prebiotics, probiotics, synbiotics, and the immune system: Experimental data and clinical evidence. Curr. Opin. Gastroenterol. 2015, 31, 153–158, doi:10.1097/MOG.0000000000000151.
- Rajkumar, H.; Kumar, M.; Das, N.; Kumar, S.N.; Challa, H.R.; Nagpal, R. Effect of Probiotic Lactobacillus salivarius UBL S22 and Prebiotic Fructo-oligosaccharid on Serum Lipids, Inflammatory Markers, Insulin Sensitivity, and Gut Bacteria in Healthy Young Volunteers: A Randomized Controlled Single-Blind Pilot Study. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 289–298, doi:10.1177/1074248414555004.
- Cozzolino, M.; Vitagliano, A.; Pellegrini, L.; Chiurazzi, M.; Andriasani, A.; Ambrosini, G.; Garrido, N. Therapy with probiotics and synbiotics for polycystic ovarian syndrome: a systematic review and meta-analysis. Eur. J. Nutr. 2020, 59, 2841–2856, doi:10.1007/s00394-020-02233-0.
- Pandey, K.R.; Naik, S.R.; Vakil, B. V. Probiotics, prebiotics and synbiotics- a review. J. Food Sci. Technol. 2015, 52, 7577–7587, doi:10.1007/s13197-015-1921-1.
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. Biomed Res. Int. 2018, 2018, doi:10.1155/2018/9478630.
- MORI, N.; KANO, M.; MASUOKA, N.; KONNO, T.; SUZUKI, Y.; MIYAZAKI, K.; UEKI, Y. Effect of probiotic and prebiotic fermented milk on skin and intestinal conditions in healthy young female students. Biosci. Microbiota, Food Heal. 2016, 35, 105–112,
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1–15, doi:10.1002/mnfr.201600240.
- Britti, M.; Roselli, M.; Finamore, A.; Merendino, N.; Mengheri, E. Regulation of immune response at intestinal and peripheral sites by probiotics. Biologia (Bratisl). 2006, 61, 735–740, doi:10.2478/s11756-006-0150-5.
- Thomas, C.M.; Versalovic, J. Probiotics-host communication. Gut Microbes 2010, 1, 148–163, doi:10.4161/gmic.1.3.11712.
- Bansal, S.; Mangal, M.; Sharma, S.K.; Gupta, R.K. Non-dairy Based Probiotics: A Healthy Treat for Intestine. Crit. Rev. Food Sci. Nutr. 2016, 56, 1856–1867, doi:10.1080/10408398.2013.790780.
- Wang, X.; Farnell, Y.Z.; Peebles, E.D.; Kiess, A.S.; Wamsley, K.G.S.; Zhai, W. Effects of prebiotics, probiotics, and their combination on growth performance, small intestine morphology, and resident Lactobacillus of male broilers. Poult. Sci. 2016, 95, 1332–1340, doi:10.3382/ps/pew030.
- Friedrich, A.D.; Paz, M.L.; Leoni, J.; Maglio, D.H.G. Message in a bottle: Dialog between intestine and skin modulated by probiotics. Int. J. Mol. Sci. 2017, 18, 1067.
- White, J.S.; Hoper, M.; Parks, R.W.; Clements, W.D.B.; Diamond, T.; Bengmark, S. The probiotic bacterium Lactobacillus plantarum species 299 reduces intestinal permeability in experimental biliary obstruction. Lett. Appl. Microbiol. 2006, 42, 19–23, doi:10.1111/j.1472-765X.2005.01800.x.
- Yamada, T.; Nagata, S.; Kondo, S.; Bian, L.; Wang, C.; Asahara, T.; Ohta, T.; Nomoto, K.; Yamashiro, Y. Effect of Continuous Fermented Milk Intake Containing Lactobacillus casei Strain Shirota on Fever in Mass Infectious Gastroenteritis Rest Home Outbreak. Kansenshogaku Zasshi 2009, 83, 31–35, doi:10.11150/kansenshogakuzasshi.83.31.
- Imaoka, A.; Shima, T.; Kato, K.; Mizuno, S.; Uehara, T.; Matsumoto, S.; Setoyana, H.; Hara, T.; Umesaki, Y. Anti-inflammatory activity of probiotic Bifidobacterium: Enhancement of IL-10 production in peripheral blood mononuclear cells from ulcerative colitis patients and inhibition of IL-8 secretion in HT-29 cells. World J. Gastroenterol. 2008, 14, 2511–2516, doi:10.3748/wjg.14.2511.
- Abdin, A.A.; Saeid, E.M. An experimental study on ulcerative colitis as a potential target for probiotic therapy by Lactobacillus acidophilus with or without “olsalazine.” J. Crohn’s Colitis 2008, 2, 296–303, doi:10.1016/j.crohns.2008.04.002.
- Amara, A.A.; Shibl, A. Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharm. J. 2015, 23, 107–114, doi:10.1016/j.jsps.2013.07.001.
- Mego, M.; Májek, J.; Končeková, R.; Ebringer, L.; Čierniková, S.; Rauko, P.; Kováč, M.; Trupl, J.; Slezák, P.; Zajac, V. Intramucosal bacteria in colon cancer and their elimination by probiotic strain Enterococcus faecium M-74 with organic selenium. Folia Microbiol. (Praha). 2005, 50, 443–447, doi:10.1007/BF02931427.
- Boudeau, J.; Glasser, A.L.; Julien, S.; Colombel, J.F.; Darfeuille-Michaud, A. Inhibitory effect of probiotic Escherichia coli strain Nissle 1917 on adhesion to and invasion of intestinal epithelial cells by adherent-invasive E.coli strains isolated from patients with Crohn’s disease. Aliment Pharmacol Ther 2003, 18, 45–56, doi:10.1046/j.0269-2813.2003.01638.x.
- Anukam, K.C.; Hayes, K.; Summers, K.; Reid, G. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 may help downregulate TNF-alpha, IL-6, IL-8, IL-10 and IL-12 (p70) in the neurogenic bladder of spinal cord injured patient with urinary tract infections: A two-case study. Adv. Urol. 2009, 2009, doi:10.1155/2009/680363.
- Thirabunyanon, M.; Boonprasom, P.; Niamsup, P. Probiotic potential of lactic acid bacteria isolated from fermented dairy milks on antiproliferation of colon cancer cells. Biotechnol. Lett. 2009, 31, 571–576, doi:10.1007/s10529-008-9902-3.
- Hacini-Rachinel, F.; Gheit, H.; Le Luduec, J.B.; Dif, F.; Nancey, S.; Kaiserlian, D. Oral probiotic control skin inflammation by acting on both effector and regulatory T cells. PLoS One 2009, 4, e4903, doi:10.1371/journal.pone.0004903.
- Chapat, L.; Chemin, K.; Dubois, B.; Bourdet-Sicard, R.; Kaiserlian, D. Lactobacillus casei reduces CD8+T cell-mediated skin inflammation. Eur. J. Immunol. 2004, 34, 2520–2528, doi:10.1002/eji.200425139.
- Weise, C.; Zhu, Y.; Ernst, D.; Ku, A.A. Oral administration of Escherichia coli Nissle 1917 prevents allergen-induced dermatitis in mice. Exp. Dermatol. 2011, 20, 805–809, doi:10.1111/j.1600-0625.2011.01326.x.
- Vaiserman, A.M.; Koliada, A.K.; Marotta, F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017, 35, 36–45.
- Musthaq, S.; Mazuy, A.; Jakus, J. The microbiome in dermatology. Clin. Dermatol. 2018, 36, 390–398, doi:10.1016/j.clindermatol.2018.03.012.
- Kalliomäki, M.; Salminen, S.; Poussa, T.; Isolauri, E. Probiotics during the first 7 years of life: A cumulative risk reduction of eczema in a randomized, placebo-controlled trial; Mosby, 2007; Vol. 119, pp. 1019–1021;.
- Huseini, H.F.; Rahimzadeh, G.; Fazeli, M.R.; Mehrazma, M.; Salehi, M. Evaluation of wound healing activities of kefir products. Burns 2012, 38, 719–723, doi:10.1016/j.burns.2011.12.005.
- Heydari Nasrabadi Study of cutaneous wound healing in rats treated with Lactobacillus plantarum on days 1, 3, 7, 14 and 21. African J. Pharm. Pharmacol. 2011, 5, 2395–2401, doi:10.5897/AJPP11.568.
- Jebur, M.S. Therapeutic efficacy of Lactobacillus acidophilus against bacterial isolates from burn wounds. N. Am. J. Med. Sci. 2010, 2, 586–91, doi:10.4297/najms.2010.2586.
- Tsiouris, C.G.; Tsiouri, M.G. Human microflora, probiotics and wound healing. Wound Med. 2017, 19, 33–38.
- Rumbaugh, K.P.; Griswold, J.A.; Iglewski, B.H.; Hamood, A.N. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections. Infect. Immun. 1999, 67, 5854–5862, doi:0019-9567/99/$04.00+0.
- de Oliveira, G.L.V.; Leite, A.Z.; Higuchi, B.S.; Gonzaga, M.I.; Mariano, V.S. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology 2017, 152, 1–12, doi:10.1111/imm.12765.
- Candon, S.; Perez-Arroyo, A.; Marquet, C.; Valette, F.; Foray, A.P.; Pelletier, B.; Milani, C.; Ventura, M.; Bach, J.F.; Chatenoud, L. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS One 2015, 10, 1–16, doi:10.1371/journal.pone.0125448.
- Hickson, M.; D’Souza, A.L.; Muthu, N.; Rogers, T.R.; Want, S.; Rajkumar, C.; Bulpitt, C.J. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: Randomised double blind placebo controlled trial. Br. Med. J. 2007, 335, 80–83, doi:10.1136/bmj.39231.599815.55.
- He, C.; Cheng, D.; Peng, C.; Li, Y.; Zhu, Y.; Lu, N. High-fat diet induces dysbiosis of gastric microbiota prior to gut microbiota in association with metabolic disorders in mice. Front. Microbiol. 2018, 9, 1–9, doi:10.3389/fmicb.2018.00639.
- Ravussin, Y.; Koren, O.; Spor, A.; Leduc, C.; Gutman, R.; Stombaugh, J.; Knight, R.; Ley, R.E.; Leibel, R.L. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity 2012, 20, 738–747, doi:10.1038/oby.2011.111.
- Ochoa-Repáraz, J.; Kasper, L.H. The Second Brain: Is the Gut Microbiota a Link Between Obesity and Central Nervous System Disorders? Curr. Obes. Rep. 2016, 5, 51–64, doi:10.1007/s13679-016-0191-1.
- Calvani, R.; Picca, A.; Lo Monaco, M.R.; Landi, F.; Bernabei, R.; Marzetti, E. Of microbes and minds: A narrative review on the second brain aging. Front. Med. 2018, 5, 1–11, doi:10.3389/fmed.2018.00053.
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Doraé, J. Low counts of faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189, doi:10.1002/ibd.20903.
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. J. Pathol. 1990, 162, 323–327, doi:10.1002/path.1711620408.
- Partlow, J.; Blikslager, A.; Matthews, C.; Law, M.; Daniels, J.; Baker, R.; Labens, R. Effect of topically applied Saccharomyces boulardii on the healing of acute porcine wounds: A preliminary study. BMC Res. Notes 2016, 9, doi:10.1186/s13104-016-2012-8.
- Brouwer, M.L.; Wolt-Plompen, S.A.A.; Dubios, A.E.J.; Van Der Heide, S.; Jansen, D.F.; Hoijer, M.A.; Kauffman, H.F.; Duiverman, E.J. No effects of probiotics on atopic dermatitis in infancy: A randomized placebo-controlled trial. Clin. Exp. Allergy 2006, 36,
- Sugimoto, S.; Ishii, Y.; Izawa, N.; Masuoka, N.; Kano, M.; Sone, T.; Chiba, K.; Miyazaki, K.; Ishikawa, F. Photoprotective effects of Bifidobacterium breve supplementation against skin damage induced by ultraviolet irradiation in hairless mice. Photodermatol. Photoimmunol. Photomed. 2012, 28, 312–319, doi:10.1111/phpp.12006.
- Queipo-Ortuño, M.I.; Seoane, L.M.; Murri, M.; Pardo, M.; Gomez-Zumaquero, J.M.; Cardona, F.; Casanueva, F.; Tinahones, F.J. Gut Microbiota Composition in Male Rat Models under Different Nutritional Status and Physical Activity and Its Association with Serum Leptin and Ghrelin Levels. PLoS One 2013, 8, doi:10.1371/journal.pone.0065465.
- Naruszewicz, M.; Johansson, M.L.; Zapolska-Downar, D.; Bukowska, H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am. J. Clin. Nutr. 2002, 76, 1249–1255, doi:10.1093/ajcn/76.6.1249.
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764, doi:10.1017/S0007114510004319.
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103, doi:10.1038/nature12198.
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913-916.e7, doi:10.1053/j.gastro.2012.06.031.
- Adlercreutz, H.; Pulkkinen, M.O.; Hämäläinen, E.K.; Korpela, J.T. Studies on the role of intestinal bacteria in metabolism of synthetic and natural steroid hormones. J. Steroid Biochem. 1984, 20, 217–229, doi:10.1016/0022-4731(84)90208-5.
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; Von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science (80-. ). 2013, 339, 1084–1088, doi:10.1126/science.1233521.
- Furrie, E.; Macfarlane, S.; Kennedy, A.; Cummings, J.H.; Walsh, S. V.; O’Neil, D.A.; Macfarlane, G.T. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: A randomised controlled pilot trial. Gut 2005, 54, 242–249, doi:10.1136/gut.2004.044834.
- Liu, R.; Zhang, C.; Shi, Y.; Zhang, F.; Li, L.; Wang, X.; Ling, Y.; Fu, H.; Dong, W.; Shen, J.; et al. Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front. Microbiol. 2017, 8, 1–12, doi:10.3389/fmicb.2017.00324.
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey, G.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Rastal, R.A.; et al. Prebiotics: Why definitions matter. Curr. Opin. Biotechnol. 2016, 37, 1–7.
- Valenlia, K.B.; Morshedi, M.; Saghafi-Asl, M.; Shahabi, P.; Abbasi, M.M. Beneficial impacts of Lactobacillus plantarum and inulin on hypothalamic levels of insulin, leptin, and oxidative markers in diabetic rats. J. Funct. Foods 2018, 46, 529–537, doi:10.1016/j.jff.2018.04.069.
- Jiang, S.; Mohammed, A.A.; Jacobs, J.A.; Cramer, T.A.; Cheng, H.W. Effect of synbiotics on thyroid hormones, intestinal histomorphology, and heat shock protein 70 expression in broiler chickens reared under cyclic heat stress. Poult. Sci. 2020, 99, 142–150, doi:10.3382/ps/pez571.
- Mofidi, F.; Poustchi, H.; Yari, Z.; Nourinayyer, B.; Merat, S.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: A pilot, randomised, double-blind, placebo-controlled, clinical trial. Br. J. Nutr. 2017, 117, 662–668, doi:10.1017/S0007114517000204.
- Eslamparast, T.; Poustchi, H.; Zamani, F.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled pilot study. Am. J. Clin. Nutr. 2014, 99, 535–542, doi:10.3945/ajcn.113.068890.
- Steed, H.; MacFarlane, G.T.; Blackett, K.L.; Bahrami, B.; Reynolds, N.; Walsh, S. V.; Cummings, J.H.; MacFarlane, S. Clinical trial: The microbiological and immunological effects of synbiotic consumption - A randomized double-blind placebo-controlled study in active Crohn’s disease. Aliment. Pharmacol. Ther. 2010, 32, 872–883, doi:10.1111/j.1365-2036.2010.04417.x.
- Sivamaruthi, B.S. A comprehensive review on clinical outcome of probiotic and synbiotic therapy for inflammatory bowel diseases. Asian Pac. J. Trop. Biomed. 2018, 8, 179–186, doi:10.4103/2221-1691.228000.
- Westfall, S.; Lomis, N.; Prakash, S. A novel synbiotic delays Alzheimer’s disease onset via combinatorial gut-brain-axis signaling in Drosophila melanogaster. PLoS One 2019, 14, 1–24, doi:10.1371/journal.pone.0214985.
- Ishikawa, H.; Matsumoto, S.; Ohashi, Y.; Imaoka, A.; Setoyama, H.; Umesaki, Y.; Tanaka, R.; Otani, T. Beneficial effects of probiotic Bifidobacterium and galacto-oligosaccharide in patients with ulcerative colitis: A randomized controlled study. Digestion 2011, 84, 128–133, doi:10.1159/000322977.
- Hatch, R.; Rosenfield, R.L.; Kim, M.H.; Tredway, D. Hirsutism: Implications, etiology, and management. Am. J. Obstet. Gynecol. 1981, 140, 815–830, doi:10.1016/0002-9378(81)90746-8.
- Carmina, E. Prevalence of idiopathic hirsutism. Eur. J. Endocrinol. 1998, 139, 421–423, doi:10.1530/eje.0.1390421.
- Azziz, R. The evaluation and management of hirsutism. Obstet. Gynecol. 2003, 101, 995–1007.
- Archer, J.S.; Chang, R.J. Hirsutism and acne in polycystic ovary syndrome. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 737–754, doi:10.1016/j.bpobgyn.2004.05.007.
- Franks, S. Diagnosis of polycystic ovarian syndrome: In defense of the Rotterdam criteria. J. Clin. Endocrinol. Metab. 2006, 91, 786–789, doi:10.1210/jc.2005-2501.
- Calvo, R.M.; Asunción, M.; Sancho, J.; San Millán, J.L.; Escobar-Morreale, H.F. The role of the CAG repeat polymorphism in the androgen receptor gene and of skewed X-chromosome inactivation, in the pathogenesis of hirsutism. J. Clin. Endocrinol. Metab. 2000, 85, 1735–1740, doi:10.1210/jc.85.4.1735.
- Vottero, A.; Stratakis, C.A.; Ghizzoni, L.; Longui, C.A.; Karl, M.; Chrousos, G.P. Androgen receptor-mediated hypersensitivity to androgens in women with nonhyperandrogenic hirsutism: Skewing of X-chromosome inactivation. J. Clin. Endocrinol. Metab. 1999, 84, 1091–1095, doi:10.1210/jc.84.3.1091.
- Ünlühizarci, K.; Karababa, Y.; Bayram, F.; Kelestimur, F. The investigation of insulin resistance in patients with idiopathic hirsutism. In Proceedings of the Journal of Clinical Endocrinology and Metabolism; 2004; Vol. 89, pp. 2741–2744.
- Talaei, A.; Adgi, Z.; Mohamadi Kelishadi, M. Idiopathic hirsutism and insulin resistance. Int. J. Endocrinol. 2013, 2013, doi:10.1155/2013/593197.
- Barbieri, R.L.; Smith, S.; Ryan, K.J. The role of hyperinsulinemia in the pathogenesis of ovarian hyperandrogenism. Fertil. Steril. 1988, 50, 197–212, doi:10.1016/s0015-0282(16)60060-2.
- Barrionuevo, P.; Nabhan, M.; Altayar, O.; Wang, Z.; Erwin, P.J.; Asi, N.; Martin, K.A.; Hassan Murad, M. Treatment Options for Hirsutism: A Systematic Review and Network Meta-Analysis. J. Clin. Endocrinol. Metab. 2018, 103, 1258–1264, doi:10.1210/jc.2017-02052.
- Moghetti, P.; Tosi, F.; Castello, R.; Magnani, C.M.; Negri, C.; Brun, E.; Furlani, L.; Caputo, M.; Muggeo, M. The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: Evidence that androgens impair insulin action in women. J. Clin. Endocrinol. Metab. 1996, 81, 952–960, doi:10.1210/jc.81.3.952.
- Corbould, A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J. Endocrinol. 2007, 192, 585–594, doi:10.1677/joe.1.07070.
- Smith, S.; Ravnikar, V.A.; Barbieri, R.L. Androgen and insulin response to an oral glucose challenge in hyperandrogenic women. Fertil. Steril. 1987, 48, 72–77, doi:10.1016/s0015-0282(16)59293-0.
- Mathur, R.S.; Moody, L.O.; Landgrebe, S.; Williamson, H.O. Plasma androgens and sex hormone-binding globulin in the evaluation of hirsute females. Fertil. Steril. 1981, 35, 29–35, doi:10.1016/S0015-0282(16)45254-4.
- DUNAIF, A.; GREEN, G.; PHELPS, R.G.; LEBWOHL, M.; FUTTERWEIT, W.; LEWY, L. Acanthosis Nigricans, Insulin Action, and Hyperandrogenism: Clinical, Histological, and Biochemical Findings*. J. Clin. Endocrinol. Metab. 1991, 73, 590–595, doi:10.1210/jcem-73-3-590.
- Dunaif, A.; Graf, M.; Mandeli, J.; Laumas, V.; Dobrjansky, A. Characterization of groups of hyperaiidrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J. Clin. Endocrinol. Metab. 1987, 65, 499–507, doi:10.1210/jcem-65-3-499.
- Azziz, R.; Carmina, E.; Sawaya, M.E. Idiopathic Hirsutism*. Endocr. Rev. 2000, 21, 347–362, doi:10.1210/edrv.21.4.0401.
- Escobar-Morreale, H.F.; Carmina, E.; Dewailly, D.; Gambineri, A.; Kelestimur, F.; Moghetti, P.; Pugeat, M.; Qiao, J.; Wijeyaratne, C.N.; Witchel, S.F.; et al. Epidemiology, diagnosis and management of hirsutism: A consensus statement by the androgen excess and polycystic ovary syndrome society. Hum. Reprod. Update 2012, 18, 146–170, doi:10.1093/humupd/dmr042.
- Irons, E.E. The clinical evaluation of drugs. J. Am. Med. Assoc. 1929, 93, 1523–1524, doi:10.1001/jama.1929.02710200007002.