Antibiotic-resistant infections present a serious health concern worldwide. It is estimated that there are 2.8 million antibiotic-resistant infections and 35,000 deaths in the United States every year. Such microorganisms include Acinetobacter, Enterobacterioceae, Pseudomonas, Staphylococcus and Mycobacterium. Alternative treatment methods are, thus, necessary to treat such infections. Bacteriophages are viruses of bacteria. In a lytic infection, the newly formed phage particles lyse the bacterium and continue to infect other bacteria. In the early 20th century, d’Herelle, Bruynoghe and Maisin used bacterium-specific phages to treat bacterial infections. Bacteriophages are being identified, purified and developed as pharmaceutically acceptable macromolecular “drugs,” undergoing strict quality control. Phages can be applied topically or delivered by inhalation, orally or parenterally. Some of the major drug-resistant infections that are potential targets of pharmaceutically prepared phages are Pseudomonas aeruginosa, Mycobacterium tuberculosis and Acinetobacter baumannii.
- lytic infection
- antibiotic-resistance
- Mycobacterium tuberculosis
- Acinetobacter baumannii
- Pseudomonas aeruginosa
- phage production
- magistral phage
- pulmonary delivery
- oral administration
- topical delivery
1. Phages as Pharmaceuticals
1.1. Phage Isolation and Enrichment
The key processes in phage therapy protocols are phage selection and isolation. The wrong choices can have fatal consequences [11]. Generally, two methods are used when choosing the appropriate phage for therapy: (1) A phage cocktail, such as Pyophage and Intestiphage. These preparations have a broader spectrum of activity than a single phage component and do not allow resistance to develop within a short time. (2) A pathogen-specific phage. Bacteria are isolated from the infection and tested for susceptibility to particular phages isolated previously [11].
Samples for phage isolation are taken from environments where the bacterial host can often be found, including soil, plant residues, fecal matter, wastewater and sewage (Figure 2). Phages against Shigella dysenteriae 2308 were isolated from the New York City sewage by Dubos et al. [12]. B_VpS_BA3 and vB_VpS_CA8 phages against Vibrio parahaemolyticus were isolated from sewage collected in China [13]. The vB_KpnP_IME337 phage against carbapenem-resistant Klebsiella pneumoniae was isolated from hospital sewage in China [14]. Li et al. [15] isolated 54 novel phages against the same organism from medical and domestic sewage wastewater. The newly isolated phage P545 had a relatively wide host range and strong antibacterial activity.
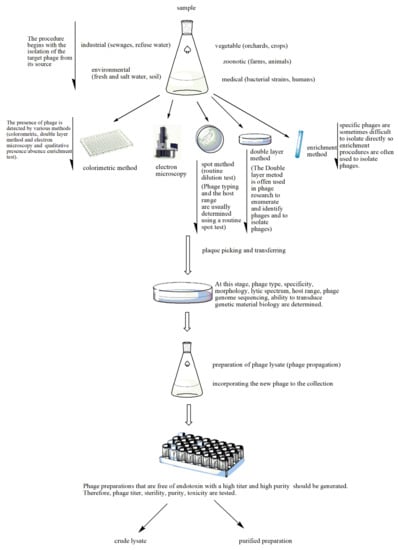
Figure 2. Stages of phage preparation. The environment (e.g., wastewater, farms and soil) is a source for all types of phages. The presence of phages in the tested sample is determined by different methods such as the double layer agar method, spot assays, the colorimetric method, the enrichment method or electron microscopy. The plaques that indicate lytic activity are picked up and transferred for the determination of phage type, specificity, etc. A phage lysate is prepared. At this stage, multiple procedures are performed to check for sterility (microbial contamination), toxicity (bacterial endotoxin or lipopolysaccharide (LPS) quantification), bacterial DNA contamination and phage titer. The purified phage preparation is stored.
Although there are differences in phage isolation, the basic principle of the methods is the same as that developed by d’Herelle, and they are generally characterized as enrichment procedures [16]. First, the presence of phages is detected in the collected sample. Selection of the bacterial host is vital for the isolation of a phage in a recently acquired sample from the environment. Solid samples are mixed with sterile broth or buffer and then subjected to centrifugation and filtration [17]. Bacteria of interest are incubated overnight with the environmental sample. The bacteria that have survived the attack of the lytic phages are removed from the mixture by centrifugation or filtration, or both. The presence of phages in the filtrate is then assessed by plaque assay or by qPCR. The isolated phages have to be analyzed for their virulence, i.e., their ability to lyse target bacteria and the range of bacterial types they are able to infect. In an alternate method, samples from the environment are plated directly onto a lawn of particular bacteria and the presence of plaques resulting from bacterial lysis is detected. The latter method has been used to discover phages that lyse Escherichia coli and various bacteria from dental plaque and the oral cavity [17,18].
In the procedure described in detail by Luong et al. [19], the target bacterial strain is isolated and incubated with the phages. Then, after several agar plaque isolations, a single plaque is cultivated overnight. The isolated phage genome is sequenced to screen and identify lysogenic and deleterious genes. Phages are grown at liter scale, and the lysate is purified to eliminate any bacteria and cellular debris by pressure-driven filtration through filters of 0.8-, 0.45- and 0.22-µm pore size, followed by cross-flow ultra-filtration to eliminate debris smaller than 100 kD. This process eliminates endotoxin, exotoxins, peptidoglycan, nucleic acids and flagella. The phage particles are purified by CsCl density gradient centrifugation and dialysis to eliminate the CsCl. Any residual endotoxin molecules are removed by lipopolysaccharide-affinity chromatography. The last step ensures that the phage preparations do not cause inflammation or endotoxic shock when administered to patients [19].
Phages are expected to be found where the host bacteria reside. For example, phages that infect intestinal bacteria can be isolated from fecal material, and phages against epidermal bacteria such as Staphylococcus aureus are most likely isolated from skin samples or wound exudates. Identifying a phage against a particular bacterium is not straightforward, however. Whereas phages that lyse antibiotic-resistant Klebsiella pneumoniae and Pseudomonas aeruginosa were readily isolated from sewage samples, phages against antibiotic-resistant Acinetobacter baumannii were not found as frequently [20]. Furthermore, phages against methicillin-resistant Staphylococcus aureus were identified only rarely.
The choice of a host for phage isolation may also depend on the ease of culturing the bacteria, as in the use of Mycobacterium smegmatis to isolate phages that will infect other Mycobacterium species. M. smegmatis grows much faster than M. tuberculosis and thus can produce a lawn on the appropriate agar surface for testing phage activity [17,20,21]. The isolated phages would then be tested further on the specific target Mycobacterium species.
Swanstrom et al. [22] investigated the variables contributing to the generation of high-titer phage stocks, using the agar layer method and coli phage T4r. The numbers of virus particles and bacteria per plate, the incubation period, the amount of soft agar in the agar layer as well as the broth volume used for virus extraction from the agar were found to be significant factors. When these factors were optimized, stock concentrations in the range of 1011–1012 infectious particles/mL could be obtained [22].
Echeverría-Vega et al. [23] used a straightforward protocol for the isolation of bacteriophages from coastal organisms. They also validated the protocol for the isolation of lytic bacteriophages for the fish pathogen bacterium Vibrio ordalii. This method has particular utility for the recovery of bacteriophages for use as natural antimicrobial agents in aquacultures. In the enrichment method, samples are added to the host produced in a suitable medium, incubated and then centrifuged. The suspension containing the phage is filtered and applied at different concentrations onto an agar medium with target bacteria. The formed plaques are then counted. Thanks to the enrichment method, phages at low concentration can reach the desired level in culture. Enrichment is an advantageous method in cases where the amount of phages is low [24]. Numerous lytic phages were isolated against Caulobacter and Asticcacaulis bacteria using the enrichment method [25]. Methods such as spot testing, plaque testing, culture lysis and the calorimetric method are used in the detection of newly isolated bacteriophages (Figure 2) [26,27,28,29,30,31]. In the spot assay [26], bacteria are grown in Luria-Bertani broth, and after they are in the early log phase, they are mixed with soft agar and poured onto a Petri plate with previously poured agar. A phage filtrate is then placed on the soft agar and the plates are incubated overnight at 37 °C, after which bacterial lysis zones are counted [28]. In the double layer agar method, a bacterial culture in the log phase is mixed with a purified phage preparation and incubated briefly to allow for phage adsorption. This mixture is combined with soft agar and poured onto a previously solidified agar layer to form a homogeneous layer. After incubation at 37 °C for 24 h, plaque formation is observed, indicating phage activity. The plaques are resuspended in Mg (SM) buffer [28].
1.2. Phage Production
Bacteriophages need a host cell to reproduce. Understanding the interactions between host bacteria and bacteriophages is a crucial step in estimating the risks in production, including possible mutations in either microorganism [32]. The production process may also be affected by the nutrient composition, oxygenation, temperature and pH [33].
The substrate and temperature chosen for phage infection and bacterial growth are important factors. Fermentation is an important stage for host bacteria to multiply and produce bacteriophages. The sterilization step is performed to destroy undesired microorganisms. The bioreactor can be sterilized with heat, medium or a combination of these. During the fermentation process, the injected air is filtered through an in-line membrane. The air released after fermentation is filtered after it condenses [34].
Phages are grown basically in shaker flasks or stirred tank bioreactors. The latter are used to carry out industrial-scale production of bacteriophages, which has been divided into three different systems: batch, semi-continuous and continuous [35]. Each system has brought about its distinct benefits and drawbacks, discussed earlier by Merabishvili et al. [36]. Mancuso et al. [37] developed a production process that makes it possible to obtain high titers of E. coli T3 phages at high concentrations (1011 PFU mL−1) using two continuous stirred tank bioreactors. The first bioreactor is just for propagation of the host bacteria at a steady-state growth rate by using controllable dilution rates and growth-limiting substrate (glucose). The second bioreactor is used for bacteriophage production and is fed from the host bacteria of the first bioreactor. Besides achieving high phage productivity of bacteriophages via the production process, the mutation risk of the host bacteria potentially caused by bacteriophages is suppressed.
1.3. Phage Purification and Quality Control
For the pharmaceutical application of phages, it is necessary to first carry out the purification process. The bacteriophage of interest is separated from host bacteria cells and debris by centrifugation, microfiltration or by using these methods together. The potential presence of any toxins in the preparations would be detrimental to the final pharmaceutical product. A Chamberland filter of 0.1–1 µm was used for bacteriophage preparations to be used in human trials [38]. It was recently clarified with a 0.2-μm filter pore size. Purification procedures of phages should follow the Critical Quality Attributes (CQA) specification [34]. The process of removing endotoxins from phages is complex because lipopolysaccharide forms micelles that have approximately the same size as phages. Therefore, extra purification methods such as ion exchange, affinity chromatography and solvent extraction are needed for lysates of phage-infected Gram-negative bacteria [33].
Endotoxin. In bacteriophage products, endotoxin measurement is critical. Gel clot, turbidimetric and chromatic methods are used for endotoxin determination in bacteriophage products. The Limulus amoebocyte lysate (LAL) assay is the most commonly used method [39].
Transmission electron microscopy (TEM). The specific morphology of phages in a final product can be viewed by transmission electron microscopy. Merabishvili et al. [40] used TEM for confirmation of the presence of the expected virion morphologic particles as well as their specific interaction with the target bacteria.
Titer. The process of determining phage concentration by dilution and plating with susceptible cells is called titering or the plaque assay. A bacteriophage capable of productively infecting a cell is named a plaque-forming unit (PFU/mL) [41].
pH. In a therapeutic formulation, the pH value is very important. According to the European Pharmacopoeia, the pH should be in the range 6.0–8.0 [42].
Nucleic Acid Contaminants. Because phages break down bacterial DNA, the presence and concentration of nucleic acid residues in final products should be determined. qPCR can be used for this purpose [33].
1.4. Phage Stability and Storage Conditions
Once solutions of phages are prepared, the biological properties of the phages have to be preserved during storage. Freeze-drying, spray-drying or encapsulation methods can be used to increase phage stability, as well as adding stabilizing additives to their solutions [43,44,45,46]. The quality, safety and storage conditions of phages to be prepared for use in treatment should be validated [47]. González-Menéndez et al. [48] investigated different preservation techniques for the storage of Staphylococcus phages (phiIPLA88, phiIPLA35, phiIPLA-RODI and phiIPLA-C1C). They evaluated the stability of phages at different temperatures (−20, −80 and −196 °C) and time periods (1, 6, 12 and 24 months). They also investigated various stabilization enhancing agents, including disaccharides, glycerol, sorbitol and skim milk. They showed that at −80 and −196 °C, all phages showed good viability after 24 months, regardless of the stabilizer [48].
1.5. Therapeutic Phages
Hyman et al. [17] proposed the following characteristics of phages to be used for therapeutic purposes: (a) The phage should be virulent and be able to cause complete cytotoxicity to the target bacterium. (b) It should be exclusively lytic and should not become temperate (i.e., lysogenic). (c) The phage should have the potential to transduce the host bacteria. (d) It should have the desired host range. (e) It should be screened for toxin genes that can affect the patient. Myoviridae, Siphoviridae and Podoviridae families are used commonly for phage therapy [49,50]. There are approximately 800 phages against pathogens such as Escherichia, Morganella, Klebsiella, Enterococcus, Pseudomonas, Staphylococcus and Salmonella [31].
2. Mycobacteriophage Therapy of Mycobacterium tuberculosis
There are more than 170 Mycobacterium species that have great variety in terms of their pathogenicity in humans [71]. In addition to M. tuberculosis, M. ulcerans and M. leprae cause Buruli ulcer and leprosy, respectively [72]. M. tuberculosis is a well-known example of an intracellular bacterium that localizes inside phagosomes of macrophages of the host and causes tuberculosis (TB), which primarily affects the lungs [73]. Multi-drug-resistant (MDR) TB cases have emerged in the late 1980s and early 1990s. These strains are resistant to the first-line drugs against TB, rifampicin and isoniazid. In 2018, the World Health Organization (WHO) reported that 484,000 new TB patients failed to respond to rifampicin. Seventy-eight percent of these patients were infected with MDR-TB [74].
Alternative treatment approaches for MDR-TB have become crucial to managing the disease. One of these approaches is mycobacteriophage therapy. More than 70 years have passed since mycobacteriophages were isolated for the first time [75]. So far, 11,282 mycobacteriophages have been isolated [76].
Bacteriophages can enter macrophages by four main routes [77] (Figure 3): (1) Endocytotic uptake of the bacteriophage alone; (2) entry into the macrophage via pathogenic bacteria together with the bound phage; (3) uptake of the bacteriophage and non-pathogenic bacteria; (4) internalization of the bacteriophage that has been encapsulated by poly-mers or liposomes. Relatively non-pathogenic vectors, such as M. smegmatis, can be used to deliver phages to the same intracellular compartments where M. tuberculosis is found [78]. The lytic mycobacteriophage TM4 was delivered in this manner to M. tuberculosis-infected RAW264.7 macrophages and reduced the bacterial counts. By contrast, the phage alone was ineffective. The administration of the M. smegmatis-TM4 complex to M. avium-infected mice significantly decreased the bacterial counts in the spleen, whereas TM4 or M. smegmatis alone had no effect [79]. The authors suggested that phage resistance (which was observed in their study) could be overcome by the use of phage cocktails.
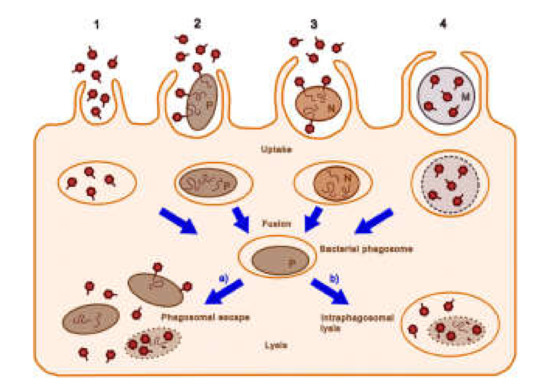
Figure 3. Possible cellular entry pathways of bacteriophages. The pathways 1–4 are described in the text. Dark red hexagons, bacteriophage; brown-filled ovals, bacteria (pathogenic bacteria, P; non-pathogenic bacteria, N); curly lines within bacteria, bacteriophage nucleic acids; orange ovals, endosomal vesicles; blue-gray circles and M, microcapsules; dotted lines, degrading bacterial membrane (reproduced with permission from Nieth et al., 2015 [77]).
The mycobacteriophage D29 was used to treat M. tuberculosis H37Rv inside RAW 264.7 macrophages [80]. The phage, administered twice over a 24-h period, caused an eight-fold reduction in the CFUs, indicating that it was able to access the intracellular compartment occupied by the bacteria. The phage was encapsulated in (or associated with) liposomes comprising phosphatidylcholine, cholesterol and Tween-80 and which were sized by extrusion through membranes of 400-nm diameter. This formulation applied to infected macrophages resulted in a two-fold improvement of the antimycobacterial effect over that of the free phage. In an in vitro model of tuberculous granuloma developed from peripheral blood mononuclear cells of patients with tuberculosis, liposomal phage was about nine-fold more effective than free D29 [81].
Aerosolized bacteriophage D29 was used to investigate the possibility of protecting mice against M. tuberculosis infection [62]. This treatment significantly decreased the M. tuberculosis counts in the lungs 1 day and 3 weeks after challenge. The authors suggested that aerosolized mycobacteriophages may be useful in conferring additional protection to healthcare workers who may be at risk of exposure to tuberculosis. D29 was also employed in a murine footpad model in treating Buruli ulcer, which is caused by M. ulcerans [82]. In infected patients, the bacterium causes necrosis of the skin, subcutaneous tissue as well as bone. If the disease reaches advanced stages, surgical resection of the skin may be necessary. The subcutaneous injection of D29 resulted in a decrease in pathology and mycobacterial counts. It also caused increased production of cytokines, including IFN-γ, in the footpads and draining lymph nodes. Endolysins are bacteriophage-encoded peptidoglycan-disrupting enzymes synthesized at the last stage of the phage life cycle in the infected bacteria [83]. One endolysin, lysine B, was found to lyse M. ulcerans infecting the footpad of experimental mice [84].
Developing mycobacteriophages into efficient therapeutic pharmaceuticals has focused on improving their uptake into macrophages and co-localization with the intracellular mycobacteria. In this process, however, it is essential to maintain the stability of the formulation and the vitality of the mycobacteriophages. In the next step, well-established in vitro and in vivo studies of effective and stable mycobacteriophage formulations are expected to translate into clinical studies with successful outcomes.
3. Bacteriophage Therapy of Pseudomonas aeruginosa
P. aeruginosa are Gram-negative aerobic bacteria classified as Gammaproteobacteria that can cause severe necrotizing bronchopneumonia, burn wound infections, urinary tract infections, otitis externa, eye infections and bacteremia [85].
In a murine model of sepsis caused by P. aeruginosa via the gut, the lytic phage KPP10 administered orally increased the survival rate from 0% in the controls to 67% [86]. The number of viable bacteria in the liver, spleen and blood were reduced in the phage-treated group, as were the levels of inflammatory cytokines in the liver and blood. Imipenem-resistant P. aeruginosa delivered i.p. resulted in bacteremia and killed 100% of experimental mice within 24 h [87]; the i.p. administration of the phage ØA392 within 1 h of infection was able to rescue all the animals. The phages were found in blood within 2 h. However, delivery of the phage at 3 h post-infection resulted in only 50% survival. In a murine burn-wound model, fatal infection by P. aeruginosa could be reduced to 87% survival when a three-phage cocktail was given i.p. [67]. The phages rapidly distributed to the blood, liver and spleen. In a similar study, i.p. delivery of multi-drug-resistant P. aeruginosa caused fatal bacteremia in mice within 2 d [88]. A phage strain that had lytic activity against numerous multi-drug-resistant P. aeruginosa given 45 min after bacterial infection resulted in 100% survival. Fifty percent of the animals could be saved even when the therapy was applied at a point where the animals were sick. The therapeutic effect of the phage was also shown not to be the result of a non-specific immune response.
When mice with acute lung infection with intranasally administered bioluminescent P. aeruginosa, which resulted in the death of all the animals within 2 days, were treated with bacteriophage PAK-P1-to-bacterium ratios of 1:1 and 10:1 via the same route, they survived until the end of the 12-day experiment [89]. Bacteriophage treatment also prevented lung infection when administered 24 h before inoculation of bacteria. Two phages were isolated from wastewater, the myovirus φNH-4 and the podovirus φMR299-2, and used to treat P. aeruginosa infection in murine lungs [90]. The pathogen was reduced by three to four orders of magnitude in 6 h. A mixture of the two phages could kill biofilms of mucoid and nonmucoid strains of P. aeruginosa on CFBE41o-cystic fibrosis bronchial epithelial cells, and the phages were shown to multiply over 24 h.
Phage GNCP treatment of multi-drug-resistant P. aeruginosa infection in diabetic and non-diabetic mice, which caused fatal bacteremia within 2 d, at a 10:1 ratio of phage:bacteria resulted in protection of 90% of diabetic animals and 100% of non-diabetic animals [91]. Bacteriophages were also effective in reducing inflammation in a murine acute infection model of P. aeruginosa [92]. The titer of phage PEV31 delivered intratracheally to mice without bacterial infection decreased with a t1/2 of about 8 h. In mice infected with P. aeruginosa, the phage titer increased by about two orders of magnitude in 16 h, and bacterial growth was suppressed, whereas it increased exponentially in the untreated animals [93].
This entry is adapted from the peer-reviewed paper 10.3390/ph14010034
